Page 14 - 林口長庚醫研部電子報-2018年9月刊
P. 14
NEWSLETTER NEWSLETTER
SEPTEMBER SEPTEMBER
2018 2018
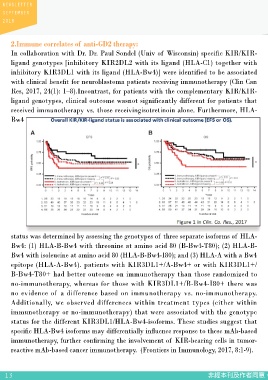
2.Immune correlates of anti-GD2 therapy:
In collaboration with Dr. Dr. Paul Sondel (Univ of Wisconsin) specific KIR/KIR-
ligand genotypes [inhibitory KIR2DL2 with its ligand (HLA-C1) together with
inhibitory KIR3DL1 with its ligand (HLA-Bw4)] were identified to be associated
with clinical benefit for neuroblastoma patients receiving immunotherapy (Clin Can
Res, 2017, 24(1): 1–8).Incontrast, for patients with the complementary KIR/KIR-
ligand genotypes, clinical outcome wasnot significantly different for patients that
received immunotherapy vs. those receivingisotretinoin alone. Furthermore, HLA-
Bw4
Reference:
1.Mody R., Naranjo A., Van Ryn C., Yu A.L., London W.B., Shulkin B.L.,
Parisi M.T., Servaes S., Diccianni M.B., Sondel P.M., Maris J.M., Park J.R.,
Bagatell R.* Irinotecan-temozolomide with temsirolimus or Dinutuximab
in children with refractory or relapsed neuroblastoma (COG ANBL1221): an
open-label, randomized, phase 2 trial. Lancet Oncology 2017, 18(7): 946-57.
2.Krbe A.K., Wang W., Carmichael L., Kim K., Mendonca E.A., Song Y., Hess
status was determined by assessing the genotypes of three separate isoforms of HLA- D., Reville P.K., London W.B., Naranjo A., Hank J.A., Diccianni M.B., Reisfeld
Bw4: (1) HLA-B-Bw4 with threonine at amino acid 80 (B-Bw4-T80); (2) HLA-B- R.A., Gillies S.D., Matthay K.K., Cohn S.L., Hogarty M.D., Maris J.M., Park
Bw4 with isoleucine at amino acid 80 (HLA-B-Bw4-I80); and (3) HLA-A with a Bw4 J.R., Ozkaynak F., Gilman A., Yu A.L.*, Sondel P.M.* Neuroblastoma patients’
epitope (HLA-A-Bw4). patients with KIR3DL1+/A-Bw4+ or with KIR3DL1+/ KIR and KIR-ligand genotypes influence clinical outcome for dinutuximab-
B-Bw4-T80+ had better outcome on immunotherapy than those randomized to based immunotherapy: a report from the Children’s Oncology Group. Clinical
no-immunotherapy, whereas for those with KIR3DL1+/B-Bw4-I80+ there was Cancer Research 2017, DOI: 10.1158/1078-0432.CCR-17-1767.
no evidence of a difference based on immunotherapy vs. no-immunotherapy. 3.Krbe A.K., Wang W., Reville P.K., Carmichael L., Kim K.M., Mendonca E.A.,
Additionally, we observed differences within treatment types (either within Song Y., Hank J.A., London W.B., Naranjo A., Hong F., Hogarty M.D., Maris
immunotherapy or no-immunotherapy) that were associated with the genotype J.M., Park J.R., Ozkaynak M.F., Miller J.S., Gilman A.L., Kahl B., Yu A.L.,
status for the different KIR3DL1/HLA-Bw4-isoforms. These studies suggest that Sondel P.M.* HLA-Bw4-I-80 isoform differentially influences clinical outcome
specific HLA-Bw4 isoforms may differentially influence response to these mAb-based as compared to HLA-Bw4-T-80 and HLA-A-Bw4 isoforms in rituximab or
immunotherapy, further confirming the involvement of KIR-bearing cells in tumor- dinutuximab-based cancer immunotherapy. Frontiers in Immunology 2017,
reactive mAb-based cancer immunotherapy. (Frontiers in Immunology, 2017, 8:1-9). 8:1-9.
13 非經本刊及作者同意,請勿做任何形式之轉載 14