|
|
|
Preparation of Mouse Embryos
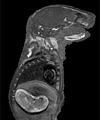
A readily available strain of mice were mated overnight, and females with a vaginal plug that next morning (indicating successful mating) were used to provide the embryos. The morning after mating was considered day .5 for determining embryonic age. At the desired age, the pregnant female mouse was euthanized, the uterus was exposed, and the embryos were carefully removed one at a time. Most of the embryos were then placed in fixative to preserve the tissues. In some embryos, the umbilical artery and vein were injected After fixation, the embryos were embedded in agarose and enclosed in an airtight capsule How the Images Were Produced Embryos were typically scanned using three-dimensional spin warp encoding with a repetition time (TR) of 200 ms, an echo time (TE) of 7 ms, and two excitations per view (NEX = 2), leading to a scan time of approx. 3.5 hours per embryo. During the magnetic resonance scan and subsequent data processing, 8.4 million voxels (256 x 256 x 128 voxels) of the embryos were sampled and then characterized by a 16-bit number representing the strength of the signal emanating from each voxel of the specimen. Each voxel of data represents a cube with equal x, y, and z dimensions The three-dimensional data were ¨volume-rendered¨ on a Silicon Graphics¤ workstation using Vital Image's VoxelView_ULTRA® to provide the whole embryo views and slice views. Volume rendering is accomplished by 1) selecting either the whole three-dimensional data set or some subset of it (slices or slabs), 2) assigning a brightness value and opacity value to each voxel based on the voxel's signal level, 3) passing "rays" through the data from back to front and allowing the rays to be attenuated according to the opacity and brightness values of the voxels encountered, and 4) displaying the Rotational movies were made by volume rendering each embryo 36 times with 10 degrees of rotation between views Magnetic Resonance Microscopy Magnetic resonance microscopy, as used to acquire these embryo images, is similar to clinical magnetic resonance imaging in its use of radio-frequency energy to non-destructively probe the specimen. However, in order to achieve the very high spatial resolution necessary to image embryos, magnetic resonance microscopy employs higher magnetic fields, stronger gradients to perturb the magnetic field, and specialized receiver coils matched to the small specimen sizes. The imaging techniques used in this work characterizes the water in the tissues of the embryo. The magnetic resonance signal is affected by factors such as the amount of water present, the rate of diffusion of water through tissues, the degree of bulk flow of water through vascular channels, and the nature of binding interactions between water and its neighboring molecules. To understand the generation of a proton-based magnetic resonance signal, it is helpful to consider the hydrogen protons as spinning tops When the protons absorb the radio-frequency energy, their precession is knocked out of alignment with the magnetic field |
| Home | Animal Embryos | Human Embryos | Embryo CD | Collaborators | Brad's Research | CIVM | Links | Contact Info |