Contents

A user’s guide
Alejandro R Jadad
5 Reporting and interpreting individual trials: the essentials
- Most reports do not contain all the information required to make informed judments on the validity of RCTs.
- Interest in the topic of a trial depends on the background of the reader and to what degree it is likely to meet immediate needs.
- Assess the likelihood of bias in a trial report using a validated scale.
- Determine whether the results of a trial are of use.
- Decide whether an RCT is important enough to be remembered and used.
- The results of individual trials should be set in the context of other relevant studies.
The extent to which you can interpret the results of a trial depends on several closely related factors, including the following:
- Your understanding of the value and limitations of RCTs as sources of information to guide decisions in health care.
- The amount and clarity of the information you find in the trial reports.
- The extent to which you are familiar with the content area addressed by the trial.
- Your understanding of the principles of data analysis and statistics.
- The time you have to read the trial report.
This book has been conceived to help you with the first two factors. This chapter will help you to identify the key elements that you should look for in a trial report to interpret its results with confidence. The other three factors - content expertise, statistical knowledge, and time - are likely to vary from reader to reader, and addressing them is obviously beyond the scope of this book.
The relationship between trial reporting and interpretation
In most cases, the only information that you will have to interpret a trial is what has been published in a journal. Unfortunately, evidence generated during the past 30 years has shown repeatedly that there is a wide gap between what a trial should report to help readers interpret their results and what is actually published.1 Therefore, you should be prepared to find that most reports do not contain all the information that you require to make informed judgments on the internal and external validity of trials.
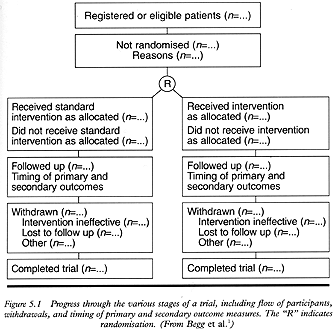
As described in Chapter 4, an international group of clinical epidemiologists, biostatisticians, and journal editors published, in 1996, a statement called CONSORT (Consolidation of the Standards of Reporting Trials),1 the aim of which is to improve the standards of written reports of RCTs and to ensure that readers find in the reports all the information that they require to interpret the trial results with confidence.2 This statement includes a checklist of 21 items and a flow diagram that authors should use to provide information on the progress of patients through a study. The original article in which the CONSORT statement was described was, however, very short and targeted authors of trial reports, not readers.1 In this chapter, I use the CONSORT statement as a template to describe the elements that you should take into account when reading a trial report to interpret its results. I also use information from my own experience as a reader, from three series of articles produced over the past 20 years by members of the Department of Clinical Epidemiology and Biostatistics at McMaster University, and from two recent books on how to read articles.3,4 The series includes articles on how to read clinical journals,5-10 users' guides to the medical literature,11,12 and a series on basic statistics for clinicians.13-16 Table 5.1 and Fig 5.1 provide the information in CONSORT's statement. If you have not read these articles, I strongly encourage you to do so. I would also like to encourage you to get a copy of the books, which are written in friendly, easy-to-follow format.
My main challenge in writing this chapter was to keep the discussion of the issues brief and clear, focusing on the essential elements that you, the reader, need to take into account when interpreting the results of an RCT report. My aim is to help you make efficient use of the limited time you can spend reading articles. When appropriate, I expand on issues discussed in previous chapters or refer you directly to the sections of the book where certain items have been discussed in more detail. In addition, I include one or more additional sources of information where you could find detailed explanations of specific issues, particularly statistical ones, which are beyond the scope of this book.
What are the key elements of a trial report needed to interpret its results with confidence?
To interpret the results of a trial with confidence, regardless of your background, the time you have available to read a trial report, and the reasons why you are reading it, you will have to answer at least the following questions:
- Is the topic interesting to you?
- Are the results likely to be unbiased?
- Would you be able to use the results?
- Are the results important enough for you to remember?
In the rest of this chapter, I try to help you answer these questions in a time-efficient manner, pointing out the sections of a report in which the information you need is most likely to be found.
I am aware that you may focus on different aspects of a report: if you are a clinician trying to decide whether to use an intervention to study or treat a particular patient; if you are a researcher designing a new RCT; if you are a peer-reviewer or an editor judging whether a trial report should be published in a journal; if you are conducting a systematic review of multiple trials; if you are deciding whether to purchase a service or a new intervention; or if you are a journalist trying to decide whether to report on an article published in the latest issue of a journal. Although I do my best to provide you with enough information to answer the above questions regardless of whom you are or why you are reading a trial report, I have focused primarily on meeting the needs of busy individuals with limited experience in methodology and research.
Now, let's try to answer the questions.
Is the topic addressed by the trial interesting to you?
The answer to this question will normally depend on your background and on the degree to which the trial is likely to meet your immediate needs. For instance, a trial entitled ‘The use of therapeutic touch for the treatment of malignant melanoma’ is likely to be interesting to you if you are an oncologist, if somebody close to you (including you, of course) has a melanoma, if you are involved in decisions about providing services for patients with melanoma, or if you are a journalist who writes about health issues.
Usually, all you need to do to establish if a report is interesting is to look at its title. If by reading the title you do not think that the article is interesting, you should either move on to another article or do something else.5 In the rare event that you cannot find enough information in the title of a report to decide whether or not it is interesting, you are likely to find such information in the first portion of the abstract or in the introduction of the report.
After reading the title of the article, if it seems interesting, you might feel tempted to jump to the conclusions in the abstract. This may occur: because of irresistible curiosity to know the ‘bottom line’ of the article; because your time is so limited that reading the title and parts of the abstract is all that you can do; or because you do not want to know anything else, even if you have enough time to read the whole article. I hope that, if you have read any of the previous chapters of this book (or any of the series on how to use the medical literature that I mentioned above), you understand that the information in the title and the abstract could give you a misleading message. You should use the information in an abstract just as an additional source of information to determine if the trial is interesting to you, unless you are reading journals containing only structured abstracts of high quality studies published elsewhere (see Chapter 7). You should resist the temptation to use only the information provided by the abstract of an original study to make decisions about patient care.
Are the trial results likely to be unbiased?
After spending a couple of minutes reading the title and the abstract of the trial report, if you decide that the article is still interesting, you should try to decide whether the trial is likely to be unbiased. This can be done in a simple manner by scoring the article using the three item validated scale presented in Chapter 4, by establishing whether group allocation was concealed at the time in which study participants were recruited into the study, and by looking at the sources of funding. Reports that receive a score of 2 points or less with the scale, or in which group allocation was not adequately concealed, are more likely to exaggerate the effects of the experimental intervention.18,19 In addition, you should remember that, if a trial has been funded by industry, the report is more likely to show beneficial effects for the product of the specific company funding the trial (see Chapter 3).20,21 As mentioned in Chapter 4, there are many other methods to assess the quality of RCTs, but none is validated, and all lack empirical evidence of their relationship to bias.22,23
It should take you approximately 3-5 minutes to assess the likelihood of bias in a trial report using the methods described above. With experience it could take you less than one minute, particularly if the authors have included methodological information in the title and the abstract. For example, if the title of the trial on therapeutic touch was ‘The use of therapeutic touch for the treatment of malignant melanoma: a randomised double-blind trial’ you could not only decide whether the article is interesting, but you could also know immediately that the article describes an RCT and you could give it 2 points for being described as randomised and double-blind.
Authors of RCT reports can help you find the information you need easily, if they follow simple approaches to make the titles of their reports as informative as possible. For instance, you will have no problem finding the information you need if the authors of the report have followed an approach that has been used extensively by journals such as ACP Journal Club and Evidence-Based Medicine, two journals that have been designed to help readers access high quality information efficiently (see Chapter 7). Using this approach, efforts are made to create informative titles that tell you something not only about the topic addressed by the trial, but also about its design and results. For instance, a more informative title for the hypothetical trial on therapeutic touch for the treatment of melanoma could be ‘Therapeutic touch as a supplement to chemotherapy can increase survival in patients with metastatic melanoma: a randomised double-blind trial’.
The abstracts of RCT reports should ideally be structured, including a systematic disclosure of the objective, research design, clinical setting, participants, interventions, main outcome measures, results, and conclusions.24 Ideally, an abstract should contain enough information to allow you to judge how scientifically sound the trial is likely to be and how relevant its results may be to your immediate needs. If the abstract is informative enough, the information it contains should be sufficient to allow you to decide whether you should read more of the article or look at another one. As I emphasised earlier, regardless of how informative an abstract is, you should resist the temptation to use only the information provided there to make decisions about patient care.
If, based on the information in the title and abstract, you think that the trial is neither scientifically sound nor relevant to you, then you should stop reading it and, if appropriate, move to another report.
On many occasions you may think that the trial is not scientifically sound, but it is very relevant to you. In these cases, the decision as to whether to read the whole report or not should depend on the amount of additional literature available on the same topic. If you are aware of other trials addressing the same issue, then you could stop reading the report you just found. If you do not know of any other trial, however, you may want to read the whole report, very carefully, and make efforts to extract as much usable information as possible. On most occasions, such unique trials should be regarded as generators of hypotheses that you, or others, could test under more rigorous conditions.
If, based on the information in the title and abstract, you think that the trial is likely to be scientifically sound and relevant, you could decide to read the whole article from beginning to end, or to read specific components of the report in a systematic manner not only to confirm whether the trial is as scientifically sound and interesting as the title and abstract suggest, but also to determine whether you could use the results and whether the results are important enough for you to remember.
How can you determine if you would be able to use the results of a trial?
RCTs, even if perfectly designed, can tell us which treatment is better, but they cannot tell us for whom it is better. How and whether to generalise the results of an individual trial to an individual patient is one of the most complex issues in health care.25 Perhaps the only situation in which you could apply the results of a trial to an individual is when you have done an n-of-1 trial on that individual (see Chapter 2). In the absence of an n-of-1 trial, you are left with information from a group of patients studied by others, in other patients, and in other settings. In these circumstances, you should try to determine the extent to which the research question of the trial matches your own questions, and how confident you feel based on the information available in the report about the execution and results of the trial.
As I have mentioned several times throughout the book, the research question is one of the most important components of a trial and its report. The research question is, however, frequently overlooked and underestimated by authors, peer-reviewers, and journal editors.
Under ideal circumstances, the report should include a clearly identified research question, which is formulated in simple terms and includes information on the broad characteristics of the participants (that is, male adults over 65 years of age), the condition (that is, metastatic melanoma), the setting (that is, a tertiary level cancer centre), the interventions (that is, chemotherapy alone versus chemotherapy plus therapeutic touch), and the outcomes (that is, disease-free survival, quality of life).26 In our example, the research question could be formulated like this:
‘What is the effect of therapeutic touch as a supplement to chemotherapy compared with surgery alone on the survival rate at 5 years of adult patients with metastatic melanoma attending a tertiary level cancer centre?’
As I said before, you will not find clearly described research questions in most of the reports you read. Not finding clearly described research questions should be one of the earliest signs of concern about the report you are reading. If you do not find a clear research question, but still think that the trial could be important to you, I would encourage you to try to find as much information as possible on the individual elements of the research question in the Abstract, Introduction and Methods section of the report.
Once you have a better idea of the question or questions that the trial tried to answer, you should look for information on how the trial was executed to establish whether its results can be used by you.
Does the report include enough information on the execution of the study?By looking for information on how the trial was executed, you will be able to judge how well it was executed and whether you could use its results. To judge the execution of a trial, you should answer the following questions.
- What was the sampling frame? How were prospective participants approached by the investigators?
The report should provide clear information on the source of prospective participants for the trial and on the methods used to approach them. These two factors are closely related to the unit of allocation. When the unit of allocation or analysis is at the individual study participant level, the sampling frame is usually a group of patients attending a given health care institution (that is, clinics, hospitals, community centres). When groups of health professionals, special interest groups, or health care institutions themselves are the units of allocation, the sampling frame is usually a geographical area. The report should mention whether all or just a subset of all prospective participants (individuals or groups) was approached. It should also describe whether prospective participants were approached consecutively, randomly, or using any other method, and provide at least the number (and ideally the number and reasons) of prospective participants who refused to be considered for the study. If the unit of analysis of the trial is not at the individual participant level, but at a group or cluster level, the authors should provide a reason for this. In addition, if the trial used cluster randomisation (see Chapter 2), you should interpret its results carefully if the authors used standard statistical techniques, because these can lead to invalid results.27
Ideally, trial reports should describe prospective participants who were invited to take part in the trial but who refused to participate. This information is, however, found very rarely in trial reports.
In addition to telling you about the sources of prospective participants, the trial report should also describe how prospective participants were approached and recruited into the study. Usual methods to recruit participants include word of mouth, an invitation letter, or an advertisement in a newspaper or other media. The report should also include information on the body responsible for approving the trial from an ethical perspective (this is usually done by an institutional review board or ethics committee).
- What were the criteria used to include prospective participants or to exclude them from the study?
The inclusion and exclusion criteria should be described in such detail that you could replicate them if you wished to do so. The description of the inclusion criteria, for instance, should provide information on the health status of the participants (that is, patients with metastatic melanoma), their previous exposure to interventions that may influence the results of the trial (that is, no previous chemotherapy, surgery, or therapeutic touch), and general demographic characteristics that could also influence the effects of the interventions (for example, age, gender, socioeconomic status, educational background, race, religious beliefs, and so on). The exclusion criteria should also be described in detail and should be justified whenever possible. The inclusion criteria are, however, often described in such detail and are so specific that a detailed description of the exclusion criteria would be unnecessary. Ideally, a report should include information on the number of prospective participants who were approached by the investigators and met the inclusion criteria but refused to participate in the study. In addition, the report should include information on prospective participants who were not included in the trial because they did not meet the inclusion criteria.After you identify the sources of trial participants (the sampling frame discussed in the previous question) and have a clear idea of the criteria used to select or exclude them, you should judge whether the sample studied is close enough to the population that will be affected by your decisions.
- Was the setting appropriate? Was it similar to your own setting?
In addition to the characteristics of trial participants, you should also examine whether the setting where they were studied resembles the setting in which you have to make decisions. Therefore, the report should include information about the place where the interventions were administered (for example, both chemotherapy and therapeutic touch were conducted in a 500 bed tertiary level cancer centre affiliated with a faculty of health sciences) and where the outcomes were assessed (for example, the outcomes were assessed in the outpatient clinics of the same institution). - What were the interventions? How and by whom were they given?
The report should include detailed information on the characteristics of the interventions (for example, the drugs and regimens used for chemotherapy, the technique for therapeutic touch given to the patients in the experimental group and the ‘placebo’ therapeutic touch given to patients in the control group), the profile of the individuals in charge of administering them (that is, the number, level of experience, and training of those performing all the interventions), and the regimens used to administer the interventions (usually, this refers to the number, size, and frequency of the doses of medication; in the therapeutic touch example, this could refer to the number, duration, and timing of the sessions). The report should also include information on important co-interventions (for example, the number of patients in each group who received adjuvant therapy) that could influence the outcomes of the trial. - How were randomisation and blinding implemented?
These two important aspects of a trial were discussed in detail in Chapter 3 and were also mentioned above in the section on assessing the likelihood of bias. In brief, the report should provide information on the method used to generate the allocation sequence, the strategies used to ensure allocation concealment, the level of blinding (masking), the methods used to blind different individuals during the trial, and whether blinding was tested. A simple way to judge whether randomisation was implemented properly is by comparing the distribution of certain characteristics of participants at the beginning of the trial (these are also called the ‘baseline’ characteristics) across the study groups. If randomisation has been successful, there should be no statistical differences among the groups in terms of baseline characteristics thought to influence the effect of the interventions. A more detailed discussion on the presentation of data on the comparability of different study groups is found elsewhere.28 - What were the outcomes of interest? How were they measured?
The report should identify all outcomes that were measured during the trial, both desirable and adverse. In addition, it should specify the tools used to assess the outcomes, the time in which the outcomes were assessed, and the profile (number and background) of the people in charge of assessing them.Once you have identified the outcomes and how they were measured, you should find out whether the authors stated which outcome was regarded as the primary one and which outcomes were regarded as secondary. The primary outcome is the main event or condition that the trial was designed to evaluate. If the primary outcome is not specified clearly or a priori (or not specified at all) and all outcomes are treated alike, there is an increased risk for the authors to highlight those with the most striking results. In addition, the more outcomes that are analysed the greater the risk of finding false positive, statistically significant results merely by chance.
-
Were the results of the trial analysed properly?
This is one of the most important, complex, and yet frequently underestimated aspects of reading a trial report. Most readers typically skip sections that include statistical information, assuming that the authors, peer-reviewers, and editors have taken care of the details and have ensured that the analyses were perfect. Unfortunately, trial reports often do not provide a complete description of the statistical methods used to analyse the results and, when they do, the methods are frequently incorrectly used and applied.29,30 Providing a detailed account of the steps and judgments that need to be made when evaluating the statistical aspects of a trial report is obviously beyond the scope of this book. Instead of overwhelming you with a myriad pieces of information that could fill a statistics book or plagiarising excellent attempts by others, I will point you to the information you should try to find in a trial report to be in a good position to judge the adequacy of the statistical analysis. With that information in hand, you could then refer to other sources that provide detailed advice, tailored to your specific needs.4-10,31,32You should look for information on:
- The type of data used to describe the results (for example, continuous, such as the time to death; or discrete, such as the number of deaths)
- The statistical tests used (for example, t-tests, c2 tests, analysis of variance, and so on)
- The level of statistical significance (the P values)
- Efforts to estimate the sample size a priori (also known as power calculation)
- The measures of the magnitude of the effects, also known as point estimates (for example, differences between the means of each group, absolute risk reduction, relative risk, odds ratio, and number needed to treat)
- The confidence intervals around the point estimates (usually you will find the 95% confidence interval)
- Intention-to-treat analyses (Chapter 3)
- Information on subgroup analyses, ideally defined a priori (males vs females; adults vs children; White vs Afro-Americans).33
- You should use this information to answer the following questions: If the results of the trial are positive, how likely are they to have occurred just by chance? If the results are negative, how likely is it that a true positive effect was missed?
- Once you have looked for, and hopefully found, information on the methods, you should look for information on the actual results of the trial.
Does the report include enough information on the results of the trial?
Be prepared to find discrepancies between what authors planned to do, what they should have done, and what they actually report in the Results section. In any case, you should expect the trial report to provide at least the following information:
- A profile of the flow of participants throughout the trial: the trial report should provide enough information for you to fill all the boxes in the flow chart of the CONSORT statement (see Fig 5.1). With this information, you will be able to judge the proportion of eligible participants who were actually enrolled in the trial (this will help you determine the representative nature of the study sample), the proportion of participants who received the interventions as allocated, the adequacy of follow up, the reasons why some participants were withdrawn from the study, and the number of participants who completed the trial.
- Description of the point estimates, measures of variability, and probability values for the main outcomes: often, you will find that trials report point estimates only in graphs, do not provide measures of variability (for example, confidence intervals, standard deviations, or standard errors), or do not give the actual probability values (for example, state that the results were ‘statistically significant’ rather than the actual value of P = 0-03).
Once you address the previous issues and determine whether you could use the results of the trial, you should try to answer the following questions.
Is the trial important enough for you to remember and use?
This is another complex question. It may, however, be simply answered by saying that the importance of a trial and its results are in the eye of the beholder. Your decision in each case is likely to depend on: the interaction between the methodological characteristics and content of the trial itself; your own beliefs, values and preferences; and the circumstances in which you are reading the article. In all cases, however, your decision will be influenced by each of the trial characteristics discussed so far, and additional factors such as your interpretation of the magnitude of the effects of the interventions, both favourable and undesirable, found in the study.
Should you base your decisions on the results of a single trial?
The answer, in most cases, is likely to be no. The main reason is that, despite how interesting, relevant, unbiased, and well reported an individual trial may be, such a trial is likely to be just one among many other studies that address the same question, and which may contradict or corroborate the findings of the trial. It is, therefore, important to set the results of individual trials in the context of other relevant studies.
In the next chapter, I introduce you to different ways in which the information from a single trial can be integrated with information from other trials.
References
1. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup D. Improving the quality of reporting of randomized controlled trialsThe CONSORT Statement. JAMA 1996;276:7-9.
2. Altman DG. Better reporting of randomised controlled trials: the CONSORT statement. BMJ 1996;313:570-1.
3. Crombie IK. The pocket guide to critical appraisal. London: BMJ Publishing Group, 1996.
4. Greenhalgh T. How to read a paper: The basics of evidence based medicine. London: BMJ Publishing Group, 1996.
5. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: I. Why to read them and how to start reading them critically. Can Med Assoc J 1981;124:555-8.
6. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: II. To learn about a diagnostic test. Can Med Assoc J 1981;124:703-10.
7. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: III. To learn about the clinical course and prognosis of disease. Can Med Assoc J 1981;124:869-79.
8. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: IV. To determine the etiology or causation of disease. Can Med Assoc J 1981;124:985-90.
9. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: V. To distinguish useful from useless or even harmful therapy. Can Med Assoc J 1981;124:1156-62.
10. Department of Clinical Epidemiology and Biostatistics, McMaster University. How to read clinical journals: VI. To learn about the quality of clinical care. Can Med Assoc J 1984;130:377-81.
11. Guyatt GH, Sackett DL, Cook DJ for the Evidence-Based Medicine Working Group. Users' guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? JAMA 1993;270:2598-601.
12. Guyatt GH, Sackett DL, Cook DJ for the Evidence-Based Medicine Working Group. Users' guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? JAMA 1994;271:59-63.
13.Guyatt G, Jaeschke R, Heddle N, Cook D, Shannon H, Walter S. Basic statistics for clinicians: 1. Hypothesis testing. Can Med Assoc J 1995;152:27-32.
14. Guyatt G, Jaeschke R, Heddle N, Cook D, Shannon H, Walter S. Basic statistics for clinicians: 2. Interpreting study results: confidence intervals. Can Med Assoc J 1995;152:169-73.
15. Guyatt G, Walter S, Shannon H, Cook D, Jaeschke R, Heddle N. Basic statistics for clinicians: 4. Correlation and regression. Can Med Assoc J 1995;152:497-504.
16. Jaeschke R, Guyatt G, Shannon H, Walter S, Cook D, Heddle N. Basic statistics for clinicians: 3. Assessing the effects of treatment: measures of association. Can Med Assoc J 1995;152:351-7.
17. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay DM. Assessing the quality of reports on randomized clinical trials: Is blinding necessary? Controlled Clin Trials 1996;17:1-12.
18. Moher D, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does the poor quality of reports of randomized trials exaggerate estimates of intervention effectiveness reported in meta-analysis? In press, Lancet.
19. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effect in controlled clinical trials. JAMA 1995;273:408-12.
20. Cho MK, Bero LA. The quality of drug studies published in symposium proceedings. Ann Intern Med 1996;124:485-9.
21. Gotszche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Controlled Clin Trials 1989;10:31-56.
22. Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Controlled Clin Trials 1995;16:62-73.
23. Jadad AR, Cook DJ, Jones AL, Klassen TP, Tugwell P, Moher M, Moher D. The quality of randomised controlled trials included in meta-analyses and systematic reviews: how often and how is it assessed? Published as: Abstract presented at the 4th Cochrane Colloquium, Adelaide, Australia, October 1996. In Review at Br Med J.
24. Haynes RB, Mulrow CD, Huth EJ, Altman DG, Gardner MJ. More informative abstracts revisited. Ann Intern Med 1990;113:69-76.
25. Bailey KR. Generalizing the results of randomized clinical trials. Controlled Clin Trials 1994;15:15-23.
26. Richardson W, Wilson M, Nishikawa J, Hayward RSA. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:12-13.
27. Donner A, Brown KS, Brasher P. A methodological review of non-therapeutic intervention trials employing cluster randomization, 1979-1989. Int J Epidemiol 1990;19:795-800.
28. Altman DG. Comparability of randomized groups. Statistician 1985;34:125-36.
29. Evans M, Pollock AV. Trials on trial: a review of trials of antibiotic prophylaxis. Arch Surg 1984;119:109-13.
30. Gardner MJ, Bond J. An exploratory study of statistical assessment of papers published in the British Medical Journal. JAMA 1990;263:1355-7.
40. Altman DG. Practical statistics for medical research. London: Chapman & Hall, 1991.
41. Streiner DL, Norman GR. PDQ epidemiology, 2nd edn. St Louis: CV Mosby, 1996.
42. Oxman AD, Guyatt GH. A consumer's guide to subgroup analysis. Ann Intern Med 1992;116:78-84.
| Table 5.1 Consolidation of Standards for Reporting Trials - CONSORT3,4 | ||||
| Heading | Subheading | Descriptor | Was it reported? | On what page no.? |
| Title | Identify the study as a randomised trial7 | |||
| Abstract | Use a structured format8,9 | |||
| Introduction | State prospectively defined hypothesis, clinical objectives, and planned subgroup or co-variate analyses10 | |||
| Methods | ||||
| Protocol | Describe | |||
| Planned study population, together with inclusion/exclusion criteria | ||||
| Planned interventions and their timing | ||||
| Primary and secondary outcome measure(s) and the minimum important difference(s), and indicate how the target sample size was projected2,11 | ||||
| Rationale and methods for statistical analyses, detailing main comparative analyses and whether they were completed on an intention-to-treat basis12,13 | ||||
| Prospectively defined stopping rules (if warranted)14 | ||||
| Assignment | Describe | |||
| Unit of randomisation (for example, individual, cluster, geographical)15 | ||||
| Method used to generate the allocation schedule16 | ||||
| Method of allocation concealment and timing of assignment17 | ||||
| Method to separate the generator from the executor of assignment17,18 | ||||
| Masking (blinding) | Describe mechanism (for example, capsules, tablets); similarity of treatment characteristics (for example, appearance, taste); allocation schedule control (location of code during trial and when broken); and evidence for successful blinding among participants, person doing intervention, outcome assessors, and data analysts19,20 | |||
| Results | ||||
| Participant flow and follow up analysis | Provide a trial profile (see Fig 5.1) summarising participant flow, numbers, and timing of randomisation assignment, interventions, and measurements for each randomised group3,21 | |||
| State estimated effect of intervention on primary and secondary outcome measures, including a point estimate and measure of precision (confidence interval)22,23 | ||||
| State results in absolute numbers when feasible (for example, 10/20, not 50%) | ||||
| Present summary data and appropriate descriptive and inferential statistics in sufficient detail to permit alternative analyses and replication24 | ||||
| Describe prognostic variables by treatment group and any attempt to adjust for them25 | ||||
| Describe protocol deviations from the study as planned, together with the reasons | ||||
| Comment | State specific interpretation of study findings, including sources of bias and imprecision (internal validity) and discussion of extemal validity, including appropriate quantitative measures when possible | |||
| State general interpretation of the data in light of the totality of the available evidence | ||||
| From Begg et al.1 | ||||
|
Home | Contents | Foreword | Introduction | Acknowledgments | How to order
© BMJ Books 1998. BMJ Books is an imprint of the BMJ Publishing Group. First published in 1998 by BMJ Books, BMA House, Tavistock Square, London WC1H 9JR. A catalogue record for this book is available from the British Library. ISBN 0-7279-1208-9
![]()
