實證醫學進行
實證醫學的實行五個步驟﹝Five steps to practice EBM﹞包括:
Step 1. Converting the need for information (about prevention, diagnosis, prognosis, therapy, causation, etc.) into an answerable question.
Step 2. Searching the best evidence with which to answer that question.
Step 3. Critically appraising the evidence for its validity (closeness to the truth), impact (size of the effect), and applicability (usefulness in our clinical practice).
Step 4. Integrating the evidence with our clinical expertise and patients’ unique biology, values and circumstances.
Step 5. Evaluating our effectiveness and efficiency in executing steps 1-4 and seeking ways to improve them both for next time.
換句話說即是按下列五個步驟來進行:
- Asking an answerable question﹝提出可回答的臨床問題﹞
- Tracking down the best evidence﹝搜尋最佳實證文獻資料﹞
- Critical appraisal﹝謹慎的文獻評讀﹞
- Integrating the appraisal with clinical expertise and patients’ preference[臨床應用]
- Evaluation the effectiveness and efficiency in executing steps 1-4﹝評估改善﹞
步驟一Asking Answerable Clinical Questions
Converting the need for information (about prevention, diagnosis, prognosis, therapy, causation, etc.) into an answerable question. 將個別病患的臨床情形提出具體可回答的問題。﹝asking an answerable question﹞
臨床問題可以分為兩種:“Background” question and “Foreground” question。一個良好的臨床問題結構如下﹝Well-built clinical question﹞:
“Background” question
1. Ask general knowledge about a disorder.
2. Have two essential components:
a. A question root (who, what, why, when…) with a verb
b. A disorder, or an aspect of a disorder
“Foreground” question
1. Ask for specific knowledge about managing patients with a disorder.
2. Have four (or three) essential components:
a. Patient and/or problem
b. Intervention (treatment)
c. Comparison intervention
d. Clinical outcomes
There are four elements of a well-formulated question (PICO)
完整描述四項目- Patient, intervention, comparison, and outcome.
- Patient ~ Who is the patient or what is the problem being addressed?
- Intervention ~ What is the intervention?
- Comparison ~ What are the alternatives?
- Outcome ~ What are the outcomes?
步驟二Searching the Best Evidence
尋找相關的醫學證據﹝包括各種文獻及醫學資料庫,發表及未發表的研究成果﹞。目前搜尋最佳實證資料,一是使用原始論文資料庫﹝primary journals or databases﹞如Medline, NEJM,二是直接使用經過整理的實證醫學資料庫 (secondary journals or databases),建議先直接使用下列四個經過整理的實證醫學資料庫﹝CDSR, CCTR, DARE, ACP journal club﹞,萬一這些都找不到,可以從Medline等資料庫中搜尋。盡量搜尋與病人問題相同且證據等級﹝*level of evidence﹞較高的文獻,再謹慎的評讀與評估其在此病人問題的適用性。
*level of evidence (The evidence pyramid圖): 在實證醫學的觀念裡,文章是有階級的(hierarchy),亦即systematic review和meta-analysis的文章最有價值,single randomized controlled trial次之,其他類型的文章又次之,我們在搜尋文獻時也運用這個的觀念,先由重要的文章看起。

實證醫學的四個主要資料庫:
1. ACP Journal Club: 含括「ACP Journal Club」(American College of Physicians,美國內科醫師學會出版)與「Evidence-Based Medicine」(ACP 與British Medical Journal Group合作出版)兩種出版品,每月至少過濾50種以上核心期刊,搜尋最佳之原始與評論性文章,結構化整理出其中重要實證所得。
2. CDSR: Cochrane Database of Systematic Reviews 為「Cochrane 合作研究機構」(Cochrane Collaboration)所出版,其為一個人與機構共同組成之國際性網路組織,有系統的研究上百種期刊文獻,專門從事有系統的評論儲備、維護和傳遞影響醫療保健相關之業務主題性評論。
3. DARE: Database of Abstracts of Reviews of Effectiveness 收錄評論性文章的全文型資料庫,由 National Health Services' Centre for Reviews and Dissemination(NHS CRD)組織出版,此一組織針對部份經過評估、挑選有學術價值的醫學期刊中選出系統性評論的文章,並將之集合而成 DARE。
4. CCTR:Cochrane Central Register of Controlled Trials 超過 300,000筆有關健康保健的控制實驗樣品參考型書目資料,內容包括 RCT〈Randomized Controlled Trials〉及 CCT 〈 Clinical Controlled Trials 〉。由Cochrane groups 及其單位組織將 Medline 及 EMBASE 檢索出來的隨機樣品文獻登記集中而成。
步驟三 Critical Appraisal the Evidence
Critically appraising the evidence for its validity (closeness to the truth), impact (size of the effect), and applicability (usefulness in our clinical practice). (VIP)
評估各種醫學報告的可信度、影響程度及臨床可使用性,並作成結論。
Validity (closeness to the truth): chance (p value, power, confident interval), bias (selection, measurement, recall bias)?
Impact (size of the effect): NNT, NNH (ARR, ARI, RRR…)
Practice applicability (usefulness in our clinical practice)
Impact (size of the effect):計算NNT (Number needed to treat), NNH (Number needed to harm)的病人數目﹝及其95%信賴區間﹞
ARR (absolute risk reduction):絕對風險比率差(風險比率差異之絕對值),實驗組和控制組產生不同結果比率之間的差異,其算法為:ARR = |EER-CER|
RRR (relative risk reduction):相對風險比率差,實驗組和對照組間產生的風險比率所降低的相對百分比。其算法為:RRR = |EER-CER|/CER
Number needed to treat (NNT = 1/ARR):需要被治療的病人數目,絕對風險比率差異值的倒數(1/ARR),即使一位病人達到實驗組治療之有益結果(或預防產生一個不良結果)所需治療的病人數目。
Number needed to harm (NNH = 1/ARI):需要被傷害的病人數目。除了考慮治療的好處外,也要考慮治療帶來的壞處,當病患接受了實驗組的治療後,可能會有病人產生副作用,亦即對多少病人數目進行實驗組療法,與對照組做比較後,會有多一個病人產生不良副作用。其算法為NNH=1/ARI。
Number needed to treat (NNT)=1/ARR: The number of patients that need to be treated to prevent one bad outcome or get one good outcome.
ARR (Absolute risk reduction) = | EER (Experimental Event Rate) - CER (Control Event Rate) | 絕對值、Number needed to treat, NNT=1/ARR(增加一位病患得到某種處置好處所需的治療病人數)、相對危險度減少百分比(relative risk reduction,RRR)
例如:Occurrence of diabetic retinopathy at 5 years among insulin-dependent diabetic in the DCCT trial.
Usual insulin regimen (CER: control event rate): 38%
Intensive insulin regimen (EER: experimental event rate): 13%
Risk reduction (calculation)
Absolute risk reduction (ARR) = CER-EER = 38%-13% = 25%
Relative risk reduction (RRR) = CER-EER/CER = 25%/38% =66%
Number needed to treat (NNT)= 1/ARR = 1/25% = 4 patients
Number needed to harm (NNH)=1/ARI:The number of patients that need to be treated to cause one bad outcome (being harmed)
絕對危險度增加百分比(absolute risk increase,ARI)= | EER (Experimental Event Rate) - CER (Control Event Rate) | 、Number needed to harm, NNH=1/ARI(增加一位受試者罹患某種醫源性傷害的治療病人數)
例如:The proportion of patients with at least one episode of symptomatic hypoglycemia.
Usual insulin regimen (CER: control event rate): 23%
Intensive insulin regimen (EER: experimental event rate): 57%
Risk increase(calculation)
Absolute risk increase (ARI) = EER - CER = 57% - 23% = 34%
Relative risk increase (RRI) = EER–CER/CER = 57% - 23%/23% = 148%
Number needed to harm (NNH)=1/ARI = 1/0.34= 3 patients (病人數目取整數)
95%信賴區間
我們常應用的P值,是比較兩組是否有顯著的差異,但只要樣本數夠大,就算只有些微差異也變得顯著,統計學上的顯著不一定代表臨床上有重大意義。許多統計學家認為95%信賴區間(Confidence interval, CI)比較有意義,該範圍代表有95%的機會涵蓋真正的療效。CI的寬度代表該研究的精確度(precision),如果CI越窄,代表我們越有信心評估治療的療效。保守的說,如果研究顯示該治療的確有顯著療效,且CI的下限仍有臨床意義,則可確定該治療具有重要的臨床價值。
Confidence interval (CI):信賴區間或信賴範圍,指在某一信賴程度內,由樣本統計量所求出預期可以包括母群體的範圍,即用以評估實驗的不確定性的指標。 信賴區間通常設為95%,這個數值的範圍代表我們對於母群體的推估具有確信95%為真的信心。
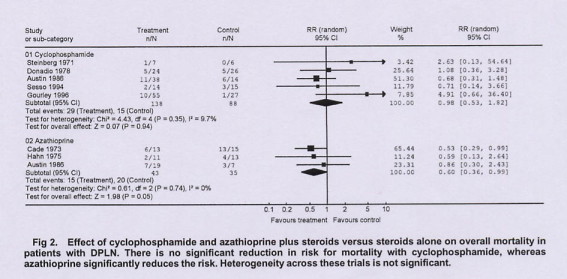
Forest plot (Meta-analysis)
This is a forest plot, with a vertical line at 1.0 representing equivalence in risk for an outcome with experimental and control treatment (null hypothesis). The RR for each outcome and its 95% CI are indicated by a solid square and a line. The size of the solid square represents the contribution (weight) of the trial to the analysis. Diamond-shaped symbols represent the summary estimator of overall effect pooling the weighted effect of individual RCTs.
Values of RR less than 1 indicate a reduction in risk for the outcome with the experimental treatment. Conversely, values of RR more than 1 indicate an increase in risk. The 95% CIs are a measure of variability in the precision of the RR estimate and its statistical significance. Heterogeneity of treatment effects between studies was investigated by visual examination of graphic meta-analysis plots and from the Cochran Q (heterogeneity chi-square) and I2statistic.
步驟四 臨床應用
Integrating the appraisal with clinical expertise and patients’ preference
將結論應用於實際患者的治療。
當有了一個可信的結果,接下來要去評估這個結果的臨床意義。科學上的文章常以RRR (Relative risk reduction)來表示療效,但臨床上以NNT來表達更為直接。
評估文章的可信度和實用性,應注意研究選入病人的條件,治療追蹤統計的方法,以及結果的定義,也應注意:
- 這些病人的分組是隨機分派的嗎?
- 分派的方法是否保密?
- 分派的兩組在治療開始時的baseline是否相似?若不相似,是否用統計的方法來修正,或增加被研究者的數目,這些代表隨機分配的方法是否適當?
- 除了研究治療項目以外,其他的治療在各組間是否相同?
- 治療方法對病患、醫護人員、研究者是否blinded(non-blinded, single blinded, double blinded, or triple blinded)?
- 追蹤是否完整?
- 分析時是否利用intention-to-treat analysis分析?
當我們在閱讀每一篇文章時,要注意是否符合這些基本原則,若是沒有,為什麼沒有,對於結果有沒有影響?另外還要考慮這樣的outcome對病人實際上的意義為何?重不重要?
步驟五 Evaluation
Evaluating our effectiveness and efficiency in executing steps 1-4 and seeking ways to improve them both for next time.﹝評估改善﹞
評估治療的效果,並且使執行上述各步驟的效率提高,能更迅速地找到解決問題的最佳方案。
﹝EBM常用字彙請查網頁查詢右下方的glossary連結﹞ |