| Laparoscopic Pelvic Lymphadenectomy |
|
Pelvic lymphadenectomy has become an integral aspect of the diagnostic and staging management of patients with adenocarcinoma of the prostate. Combined with laparoscopy, it has become a popular procedure. Its sole purpose is to identify occult nodal metastasis which could upgrade the staging of these patients and change their management.
Diagnostic imaging modalities such as lymphangiography and CT scans are notoriously unreliable for the evaluation of malignant invasion of nodal tissue. In 1997, the recovery data from laparoscopic lymphadenectomy has been shown to be superior to the open procedure while the cancer detection rate remains equal for each procedure. If Danella's criterion are used (PSA > 40ng/dl, Gleason Score >8 or Stage B2, C or D0) the indications of this procedure are becoming very limited and only applicable to a small number of patients. However, these indications used in clinical practice still vary widely from one institution to the other.
In the first edition of this book, we described two types of pelvic lymphadenectomy: 1. The limited pelvic lymphadenectomy or obturator chain dissection, 2. The extended pelvic lymphadenectomy which includes an obturator and iliac chain dissection. Currently, most surgeons perform a limited lymph node dissection or an hybrid dissection involving some of the nodal tissue cephalad to the region.
| UNDERSTANDING THE ANATOMY |
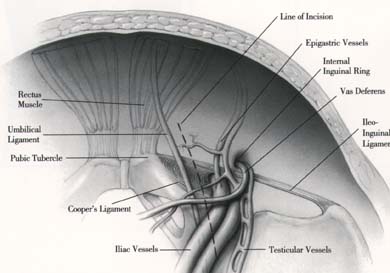
As in the laparoscopic repair of inguino-femoral hernias, understanding the anatomy of the inguino-femoral region is essential (Refer to Laparoscopic Inguinal Hernia Repair). Major complications can occur when surgeons unfamiliar with this laparoscopic approach initiate this procedure.

| THE TECHNIQUE |
The laparoscopic pelvic lymphadenectomy is indicated in the following patient groups with adenocarcinoma of the prostate:
- Candidates for a radiooncologic or surgical resection protocol with PSA >40 ng/dl or Gleason Score >7 with PSA >15 ng/dl
- Suggestion of advanced local disease
- Candidates for radical surgical resection at high risk for malignant invasion of nodal pelvic tissue.
STEP 1: The Peritoneal Incision and Clipping of the Vas Deferens
A long peritoneal incision is made with the ENDO SHEARS* instrument. Great attention should be given to the iliac vessels. The incision is continued upward and parallel to the umbilical ligament. The vas deferens is encountered, dissected and clipped using the ENDO CLIP* 5 mm. It is then divided. The incision is continued over the iliac vessels and a peritoneal flap is raised bluntly. Again, great care should be taken not to injure the iliac vessels.
STEP 2: Exposing the Obturator Fossa
The obturator fossa is now exposed. The landmarks that must be seen are:
- Laterally, the external iliac vessels
- Medially, the obturator nerve and vessels, which form a V with the external iliac vessels
- Distally, the circumflex vein or the ileo-inguinal ligament
STEP 3: Resecting the Nodal Tissue
The nodal bearing tissue to be resected occupies the entire obturator fossa. However, some of this tissue continues behind the external iliac vein and will have to be resected.
Using a blunt probe and the atraumatic grasper, the external iliac vein is retracted laterally. The lymph tissue is grasped with atraumatic graspers and dissected from the medial aspect of the vein.
It is crucial for the operator to identify an aberrant obturator vein if it is present. If injured, it would create significant and difficult to control bleeding.
The entire nodal tissue between the obturator nerve and the external iliac vein is resected. The NEDO CLIP* Applier is used to facilitate dissection and minimize bleeding. The circumflex vein is the distal limit of the dissection. Occasionally it will have to be ligated and cut to facilitate the dissection.
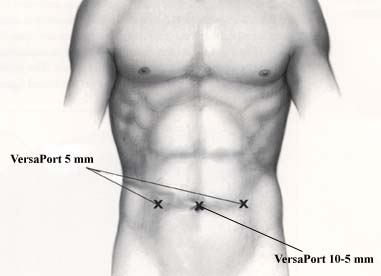
The nodal tissue is then amputated en bloc from the obturator fossa. It can be removed with an ENDO CATCH* instrument or with a 10 mm Morcilator inserted via the 10 - 5 mm VERSAPORT* trocar. We no longer close the peritoneum. Hemostasis is checked and the contralateral side is done in the same fashion.
STEP 4: Extended Pelvic Lymphadenectomy
In this variation, additional nodal tissue is resected cephalad to the obturator fossa. The beginning of this procedure is identical to the limited lymphadenectomy as previously described. The only difference is the proximal end of the incision over the obturator fossa is extended upward a few centimeters.
The nodal tissue to be resected will include the obturator fossa tissue and will extend to the iliac bifurcation (between the ureter and the iliac vessels). All vascular branches should be clipped not electrocoagulated. The operator should be careful not to injure the ureter.
| THE FUTURE |
With marked improved recovery and technical ease, the laparoscopic lymphadenectomy has become a definite asset in the management of patients with adenocarcinoma of the prostate.
References: