�� ��
|
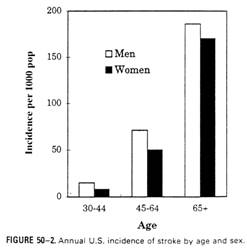
Stroke
rehabilitation
-------------------------------------------------- |
P1 |
|
Traumatic
brain injury rehabilitation
------------------------------- |
P12 |
|
Spinal cord
injury rehabilitation
------------------------------------ |
P28 |
|
Electrodiagnosis ------------------------------------------------------ |
P37 |
|
Pediatric
rehabilitation
----------------------------------------------- |
P45 |
|
Musculoskeletal
ultrasound
----------------------------------------- |
P59 |
Stroke rehabilitation
Definition
��a
nontraumatic brain injury caused by occlusion or rupture of cerebral blood
vessels
�� results in sudden neurological
deficit
��losss of motor control,
��altered sensation,
��cognitive or language impairment,
��disequilibrium,
�� coma
Pathological classification of
stroke
��Hemorrhagic:
15%
��Intracerebral: 10% --HTN, AVM, tumor
��Subarachnoid: 5% -- aneurysm rupture
��Ischemic: 85%
��Large vessel: 40% --artherosclerotic à large and small vessels thrombosis.
nSmall vessel: 20% -- HTN
��Cerebral embolism: 20% -- cardiac origin
��Less common cause: 5% -- vasculitis, hypoperfusion
Temporal classification of stroke
��TIA: an event in which
neurological symptoms develop and disappear over several minutes and completely
resolve within 24 hours
��Artherosclerotic carotid disease à
urgent evaluation and preventive treatment.
��RIND: a transient neurological event that lasts longer than 24
hours
��Infrequent, unknown etiology,
 ��Stroke in evolution: unstable ischemic event, progressive
development of more severe neurological impairment
��Stroke in evolution: unstable ischemic event, progressive
development of more severe neurological impairment
��Complete stroke
When to start rehabilitation
When a patient��s neurological and
medical status is stable.
Stroke risk factors
��Hypertension:
most important----Leading risk factor of stroke and CAD
��Heart disease and chronic atrial
fibrilation
��TIA, asymptomatic carotid bruit
��Smoking
��Hypercholesterolemia
��Obesity
��Stroke history
Stroke pathophysiology
��Ischemic
stroke
��Thrombosis
��Embolism
��Lacunes
��Hemorrhage
stroke
��Intracerebral hemorrhage
��Subarachonid hemorrhage
��hydrocephalus
Stroke related impairments
��Motor
control and strengthen
��Motor coordination and balance
��Spasticity
��Sensation
��Language and communication
��Apraxia
��Neglect syndrome
��Dysphagia
��Uninhibited bladder and bowel
Motor control and strength
��Anatomy
��Primary motor area: precentral gyrus
��Topography of precentral gyrus
��Cortex�Xinternal capsule�Xpyramidal tract(brain stem)�Xcorticospinal
tract(spinal cord)
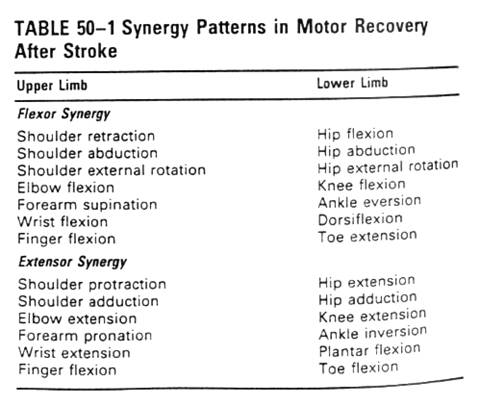
��Recovery
��Weakness and poor control of voluntary movement, reduced muscle
tone
��Recover: nonfunctional mass flexion and extension of the
limbs(synergy, mass contraction) -> independent of synergy
Motor coordination and balance
��Extrapyramidal system..
��Anatomy:
��Premotor area, anterior to precentral
gyrus: motor planningà int. capsule ant.limb àbasal ggl and cerebellum
��Input from visual, vestibular, and
somatosensory
��If damageà static/dynamic balance impaired as ataxia, chorea, hemiballismus,
tremor
Spasticity
��Velocity-dependent
increased in resistance to muscle stretch that develops after an UMN injury
within the CNS
��Loss of upper motor neuron control à disinhibited �\and �^motor neuron activity and ��sensitivity to Ia and II muscle spindle afferent fiber à hyperactive spinal reflex
��Both increase in tonic and phasic
reflexes
��If voluntary motor activity returns à
reduction in tone and reflex response is noted. But incomplete
Sensation
��Loss
of joint and skin protection, balance, coordination and motor control
��Hypoesthesia
��Severe pain occasionally in spinothalamic tract or thalamus à TCA, anticonvulsant, ES
��Anatomy
��(pain/temperature) dorsal root ggl à spinothalamic
tract à
VPL n. of thalamus à primary sensory cortex
��(proprioception/stereognosis) à dorsal root ggl à
dorsal column à
nucleus gracilis/cuneatus à medial lemniscus à VPL n. of
thalamus à
primary sensory cortex(postcentral gyrus)
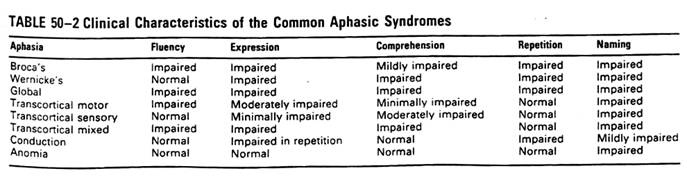
Language and Communication
��Testing: oral expression, verbal
comprehension, naming, reading, writing and repeating
��Language: left or dominant hemisphere
��Prosody: non-dominant hemisphere
��Rhythmic pattern and vocal intonation
of speech that adds emphasis and emotional content to language
Management
Acute stroke management
��Cerebral
flow
��normal cerebral autoregulation: 50 mL/100g/min
��20 mL/100g/min: normal neural activity can maintain
��10-20 mL/100g/min: electrically silent(energy dependant Na-K pump
fails), basilar cellular function are supported
��< 10 ml/100g/min: cellular death
��Ischemic penumbra: Surviving but
inactive neural cells area located at the rim of the ischemic injury
�����ɶ��V�[�V���Ϭ�
��National Stroke Association suggest acute stroke management within
first 6 hrs.
The first 6 hours
��Thrombolytic agent
��rt-PA(recombinant
tissue plasminogen activator: NINDS study: reduced unfavorable outcome in
selected patients who present to the emergency department within the first 3
hours after acute stroke onset FDA�įǦ�����s
��IV
0.9 mg/kg
��IV Streptokinase:
unacceptable ICH rate and mortality
��Intra-arterial
infusion: Pro-urokinase: acceptable safety,
��Heparin:
��IV
heparin, usually used, but little evidence of its efficacy and guidelines of
use
��Cardioembolic
stroke 12% recurred within 3 weeks à heparin àwarfarin
��Many
clinician advocate use heparin > 48 hrs to prevent hemorrhagic
transformation of infarcts.
��Calium channel
blocker(Nimodipine): for SAH vasospasm --> routine for 21 days
��Not
effective in ischemic stroke
��NMDA antagonist:
potential protective
��During
acute stroke, glutamate and aspartate are released à NMDA receptor à
influx of cation à
cell death
��Many
study trial stopped because of safety concern and lack of effect
Emergency
department evaluation and treatment
��NINDS
recommended: Physical assessment, laboratory testing, cranial CT and CT
interpretation within 45 minutes
��Lab: electrolyte, Bun/Cr, glucose, cholesterol, CBC with platelets
count, PT, aPTT, ESR, UA
��Brain CT: to identify ischemic VS hemorrhage
��Negative for the first 24~48 hrs, in
ischemic stroke except:
��Loss of definition of gray-white
matter junction, sulci effacement, bright
area in the MCA main stem
��ICH: hyerdensityàbecomes hypodense within 1~2 weeks,à resolution with residual small hypodense area
��ICH of basal ggl. and thalamusàhypertension related
��IVH or SAHàaneurysm and AVM related
Acute medical management
��Airway
management
��IICP (cerebral edema,acute
hydrocephalus) àhyperventilation
or external ventriculostomy device (EVD)
��BP:
��Usually elevated for weeks
��Acute HTN should be treated only if: symptomatic, end-organ
injury, DBP> 120 mmHg
��When treated, SBP maintain 150/DBP maintain 90 mmHg
��Ca-blocker not ideal because it may cause vasodilatation of
cerebral vessel à
further IICP and poor cerebral perfusion
��Better choice: mixed �\and �]blocker (labetolol),
�\2-receptor antagonist
(clonidine)à
less effect on cerebral perfusion
��Hyperglycemia, response to physiologic
stress associated with ��cortisol
��Animal study: ��glucose as the substrate for partially perfused area cell à ��anaerobic metabolism à �� lactateà cytotoxic, so use of insulin and strict glucose control are potentially neuroptrotective
��
Natural spontaneous neurological
recovery
��The degree of
natural recovery variable, generally, deficits decline in frequency by about
1/2-1/3
��At one year follow-up, hemiparesis(73 à37%), aphasia(36à20%), dysarthria(48à16%), dysphagia(13à4%), incontinence(29à9%)
��The
time course for recovery also variable
��Most improvements in
physical functioning occur within the first 3-6 months, but later recovery also
is commonly seen
��Recovery (two
types)
��Reduction in the
extent of neurological impairment �Vnatural spontaneous neurological recovery
��Improved ability to
perform daily functions in their environment, within the limitations of their
physical impairments
��Feed,
dress, bathe, control elimination,, walk, ADL
��rehabilitation
intervention is thought to exert the greatest effect. Even patients with no or
minimal neurological recovery significantly improved in their ability to carry
out day-to-day skills with rehabilitation.
��Most (but not all) stroke motor recovery follow up the pattern
��lower extremity
function recovers earliest and most completely, followed by upper extremity and
hand function
��return of tone
precedes return of voluntary movement
��proximal precedes distal
��mass
movement(synergy patterns) precis specific isolated coordinated volitional
motor functions
��Lower
extremity motor control recovery
��Improve of 1
Brunnstrom: 39%
��2 or more
Brunnstrom: 12 %
��Upper
extremity motor control
��1 Brunnstrom: 24%
��2 Brunnstrom: 8 %
Principles of stroke rehabilitation
��Holistic care
��including social,
vocational and economic factors
��Team
management
��interdisciplinary
team: physician, nurse, PT, OT, ST, orthotist, psychologist, social worker
��Goal-directed
treatment
��early step: establishing realistic, practical and feasible goals that are mutually agreed upon by the patient, family and professionals
��Focus on
learning and adaptation
��Rehabilitation
process consists of learning and adaptation.
��Theory of learning:
reacquire old skills or develops means to compensate for new impairements in a
logical, coherent manner.
�� ��Supervised
practice�� and�� timely support, education, reasurance, direct assistance,
immediate feedback�� are two necessary component of this learning process.
��Rehabilitation is ��the planned withdrawal of support��
��Therapy
environment
��Beneficial greatly
from practice in therapy environments that closely reflex natural home or
community settings
��Timing of
therapy
��Specific therapy
schedules should be individualized.
��The stroke
rehabilitation literature does not provides specific guideline about the amount
of therapy for each specific problems. ���L�@��{���AReasonable participate in functional activities at least once
a day
��Endurance, medical
stability, mood, motivation à�|�v�T�f�w���@����
��
Attention
to psychosocial issues
��Depend largely on
pre-stroke coping styles. Levels of frustration tolerance and ability and
mechanism used to deal with adversity
��Family issue of
emotion
��Focus of families
��Family
members serve as members of the rehabilitation team
��Participate
activity in the rehabilitation process
��Emphasis
on community issues
��Enable
smooth safe transition to community living
Rehabilitation
assessment and interventions during the acute phase
��The maximum benefit of rehabilitation
can be achieved when rehabilitation interventions area begun as early as
possible after stroke
��Early poststroke rehabilitation is ��preventive�� + ��therapeutic��
Levels of post-acute stroke care
��Acute
inpatient rehabilitation
��Traditional interdisciplinary hospital-based coordinated program
of medical, nursing and therapy.
��>= 3 hrs of therapy per day
��Need daily physician supervision and around-the-clock nursing
care.
��Subacute inpatient rehabilitation
��Less intensive program is used(�]��mild stroke or poor endurance tolerance), 1-3 hrs of therapy per
day
���H�@�h���D�����U�A24 hrs nursing care,
Dr�d�Ф@�g1-3��
��Day
rehabilitation
��3-8 hrs/day
���f�H�դѨӧ@�_���A�ߤW�^�a�Abut the program all the same
with inpatient rehab.
��A comprehensive and
coordinated program, rehab. team had regular scheduled conference.
��Outpatient therapy
���f�H�դѨӧ@�_���A�ߤW�^�a
��PT/OT/ST�U���U���Afor patient with focal deficit and need specific functional
training.
��Home therapy
��Home is the most
familiar environment, all the patient and family to learn specific functional
tasks
��But may lack support
of specific equipment and experienced staff
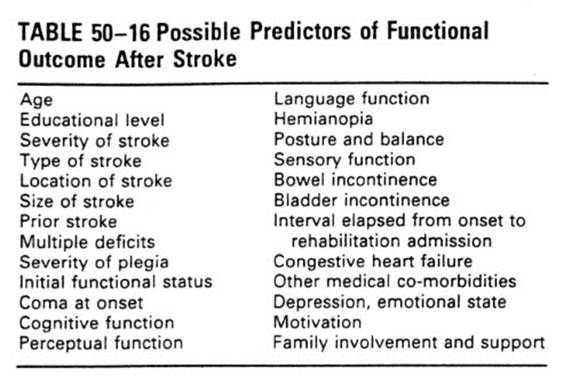
Rehabilitation outcomes
��Functional
and social outcomes
��**Physical
performance, functional abilities and quality of life got better after rehab.
��Greatest
improved in locomotion, mobility, self-care, and sphincter control improved
after rehab.
��A
report: Hospitalization FIM: average from 63 on admission to 87 at discharge.
��Less
improved in communication and social
cognition.
��In general,
75%~85% of stroke patient are discharged to home after formal acute rehab.
care.
��3/4 discharged to
community
 ��
��
1.
The importance of thorough and consistent assessment of status
at each stage of The recovery process to help guide treatment decisions and to
monitor patient progress
2.Early implementation of rehabilitation interventions during acute
care to promote recovery and prevent complications
3.Selection of the type of rehabilitation program and services best
suited to meet the patient��s needs
4.Establishment of realistic rehabilitation goals and provision of
treatment in accordance with a carefully developed rehabilitation management
plan
5.Combined follow-up and treatment during transition to a community residence
AHCPR guidelines
��Threshold
criteria of the need for rehab.:
��Medical stability
��Presence of functional deficit
��Ability to learn
��Endurance to sit up at least for 1 hr.
��Ability to participate actively in the rehab. program.
��One criteria for admission to a
comprehensive interdisciplinary program:
������2 types of disabilities, need ��2 disciplines of professionals to assist in management.
Special patient considerations
Pediatric
stroke
��Incidence:
2.55 per 100,000 children per year
��Different
presentation from adult
��Seizures, fever,
delayed achievement of development milestones
��Cause
different from adult:
�� hereditary,
congenital heart disease, metabolic disorders, coagulopathy, drugs,
intracerebral vascular anomalies.
��Prognosis:
better than adult
Stroke in young adults
��1/3 of stroke
in individuals < 65 y/o
��26% in persons 45-65 y/o
��Hemorrhage
stroke 1/3 of young adult, 1/5 of all stroke survivors.
��Ischemic
stroke/infarction
��Atherosclerosis 20%
��Cardiogenic embolism
20%
��Collagen vascular
disease 10%
��Coagulopathy 10%
��Young
stroke survey: angiography, coagulation test, collagen vascular disease
evaluation, cardiac work-up (e.g. TEE)
Geriatric stroke
��Young age at stroke onset has a
favorable effect on long-term and short-term stroke survival, BUT THE EFFECT ON
FUNCTIONAL RECOVERY IS LESS CERTAIN!
��Age may be just a marker for co-morbidities, prior stroke, limited
social supports
Traumatic Brain Injury
Terminology
nGeneral term for all injuries to
the brain caused by external force
(�x���Ҧ��~�O�y��������)
nTBI is a general term that dose
not imply a specific pathology

Non-traumatic brain injury
nA Specific etiology and
pathology
nAnoxic brain injury(=hypoxic
encephalopathy, hypoxic brain injury)
nCause: cardiac/respiratory arrest, CO
poisoning
nStroke
nInfection
nToxic-metabolic brain injury
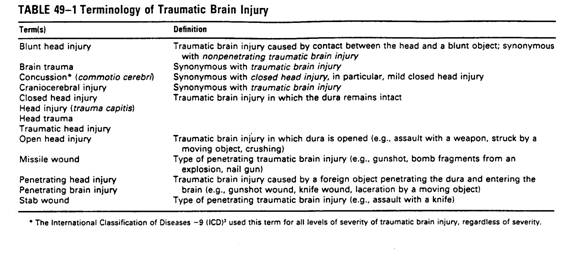
Pathophysiology �V closed or open head injury
nPrimary mechanism occurs at the moment of
impact, secondary mechanism are triggered by primary mechanism and cause additional
damage.
nBrain injury is often the summation of
effects of multiple primary and secondary mechanisms à diffuse injury, especially in closed head
injury.
nThe major mechanism are caused by
acceleration-deceleration à diffuse axonal injury, multiple
petechial hemorrhage, contusion, and cranial nerve injury.
A.
Primary injury
1.Diffuse
axonal injury (DAI)
* Acceleration-deceleration and
rotational force
*The distribution is centripetal
model, in mild TBI, cortex(surface)
is more likely injured, in severe TBI, deeper structures are injured,
like cropus callosum( longer fiber tract).
* May cause loss of
consciousness
2. Cerebral contusion
* Relative low velocity impact
* Inferior frontal lobes and
anterior temporal lobes are most frequent due to irregular bony surface.
* Focal cognitive and sensory
motor deficit, risk of
seizure

B. secondary injury
* IICP
* SDH, EDH, ICH
* Vasogenic or cytogenic brain
edema
* Herniation
* Hydrocephalus
* Brain ischemia/hypoxia
* Free radical injury,
excitotoxicity
One study: moderate hypothermia (32��~33��c) for 24 hrs, improve outcome
Damage is
located along the track of the bullet and indriven bony fragment
Cause focal damage (e.g. hemiplegia, hemianopsia)
Anoxic brain injury
nCaused by hypoxemia or decreased
cerebral perfusion.
nAlthough diffuse injury is often
noted, but selective vulnerable areas are existed.
nHippocampus, most vulnerable à amnesia
nBasal ganglion, cerebellum à movement disorder
Epidemiology
nMale > female, young
adult(18~25 y/o)�̦h
nSingle largest indirect cause:
alcohol drinking
nSingle largest external cause:
motor vehicle crash (young adult), followed by auto-pedestrian crashes
(children), falls (children and elderly person), assaults
Assessment techniques and
prognosis



nInitial used for TBI severity
classification, now also used in other brain injury resulting in conscious
disturbance
nDepth and duration of
unconsciousness measured by GCS is the single best predictor of outcome
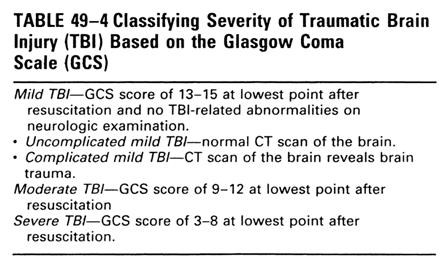
Severe TBI
nGCS: ��8, à coma, not open the eyes, no evidence of
cognition(follow command, communication)
nAccounts for large majority of TBI
inpatient rehabilitation.
nMajority have permanent neurologic
impairement and functional disabilities.
nUsually takes one year or longer to reach
maximal neurological recovery
Moderate TBI
nGCS: 9~12, combative or
lethargic, possible follow command, but not answer question appropriately(V3~4)
nMajority resumes preinjury
activity, despite few have mild cognitive deficit.
nRecovery, shorter than severe
TBI
Mild traumatic brain injury
nDefinition:
nLowest GCS�� 13, might be confused, but
awake
nMild TBI can be defined
ndespite GCS =15 if there is lesion noted by
images,
nor if the injury caused altered mental
status such as loss of consciousness, a period of confusion or disorientation,
or amnesia for the injury itself
nUncomplicated mild
nComplicated or high- risk mild TBI. àCT and/or MRI reveals brain trauma
nLoss of consciousness, if any, <= 30
mins
nPosttraumatic amnesia <= 24 hrs
nNo focal neurological deficit
nConcussion = mild TBI
nNeuroimaging findings are the single best
prognostic indicator for mild TBI.
nGenerally returns to preinjury activity
without detectable cognitive deficits.
nRecovery, usually within 3 months, most
case within 1 month
nCommon complains of mild TBI:
nCognitive: attention and concentration
difficulties, memory impairment
nAffective: irritability, depression,
anxiety
nSomatic: headache, dizziness, insomnia,
fatigue, sensory impairments
Neuroimaging of brain injury
nCT is the choice of test during the acute
stage:
nSensitivity to blood, bony fracture
nNot contraindicated with metallic material
implant
nMRI
nMore sensitive than CT(e.g. nonhemorrhage
shear injuries, some areas of contusion;)
nInferrior frontal region and brain stem
contusion or other injury may be obscured by bony defect in CT
nDisadvantage: contraindicated by metallic
material, needing long time for exam
nCT findings are related to later gross
outcome(conscious survival VS vegetative), but some studies suggested poor
correlation between it and functional outcome.
nMRI should be more useful than CT in rehab
planning( helpful in explaining patients neurologic and neuropsychological
deficits )
nSPECT(single proton emission CT) could have
an important role in evaluating unconscious or mild TBI, may be more sensitive
nEEG
nLimited usefulness in detecting
posttraumatic seizure since interictal EEG abnormalities can merely reflect the
severity of TBI
nIn acute setting, EEG is a powerful
predictor of survival from TBI.
nPoor outcome: abnormal sleep spindles and
predominance of alpha waves
nSSEP: Studies: bilateral absence of N20 to
P22 of comatose TBI patient was a strong predictor of failure to recover
consciousness
nBAEP: Studies: absence of wave V or other
components also predicts poor outcome

Summary of Acute Prognostic Indicators
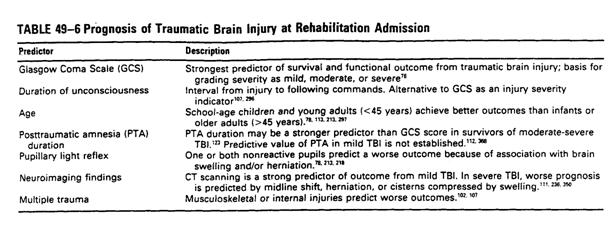

Others:
1. Glasgow-Liege Score: GCS +
brainstem reflex
2. SEP: particular sensitive and
specific
3. EEG
4. Apolipoprotein E4:
play a role in neural regeneration
studies: presence of the apolipoprotein e4 allele
(
which produces APOE-4) increase the risk of Alzheimer��s
disease and severity in certain TBI populations
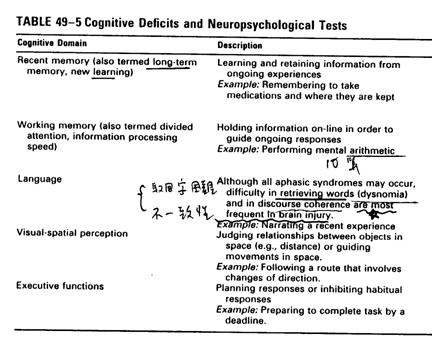
Outcomes measurements
n
nDisability Rating Scale (DRS):
from coma to community, more sensitive and comprehensive than GOS
nThe Rancho Los Amigos Levels of
Cognitive Functioning Scale: cognitive recovery
nFunctional
nFunctional Assessment Measures
(FAM): more cognitive oriented items
nCommunity Integration
Questionnaire(CIQ)
nSupervision Rating Scale(SRS)
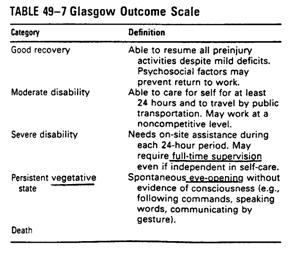

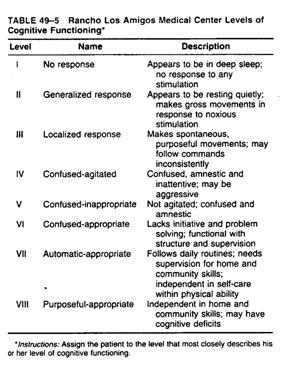

Continuum of brain injury
rehabilitaiton
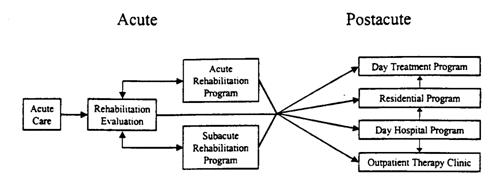

Rehabilitation during critical care
nGoal: prevent complication
nMeasure:
nPROM 2 times/day: prevent contracture
nPositioning: prevent pressure ulcer, edema,
contracture
nPrevent spasticity
nIncontinence
nNutrition
nEarly surgical treatment of orthopedic
injury
Acute brain injury rehabilitation
nInitial rehab. Evaluation à acute/subacute/or post-acute program
nMedical stable à transfer to acute program
nThe criteria of following commands to be
admitted to rehabilitation is unjustifiable.
nMajority of TBI regain consciousness(40~60%
at one year, table 49-9)
nIndication for admission to brain injury
rehabilitation units instead of general rehab. unit
nUnconsciousness/inconsistent consciousness,
agitation, risk of complication, severe cognitive deficit
Stages of neurobehavioral recovery from brain injury�V TBI patient have
long course of recovery, more functional progress
nComa/Unconsciousness/Impaired consciousness
nRehab of patient with impaired
consciousness
nPost-traumatic Amnesia and Agitation
nRehabilitation of the Agitated Patients
nRehabilitation During and After
Posttraumatic Amnesia
nPost-acute brain injury rehabilitation
Coma and unconsciousness
nComa: not open the eyes, no evidence of
cognition
nDue to disruption of ascending pathway from
deep structure to surface cortex
nDAI, brain stem or diencephalic structure
compression
nRecovery from coma(centripetal model)
nBegins with eye opening and sleep-awake
cycles à then followed commands à speak
nStudies: 10~15% still unconscious at
discharge
nAt one month, those still unconscious
patients regained sleep-awake cycles, also have pupillary
reactivity/oculocephalic reflex, primitive behavior(chewing, eye roving eye
movement), vegetative function(respiration) àall of which reflect brain stem and
hypothalamus function
Persistent vegetative state(PVS)
nWakeful unresponsiveness
nCharacterized by the presence of
spontaneous sleep-wake cycles
nAbsence of cortical activity as judged
behaviorally
nNo reproducible, purposeful, or voluntary
behavior response to stimuli
nNo evidence of language comprehension or
expression
nIt is a diagnostic term, not prognostic.
nThe Task Force defined PVS: present for one
month after an acute traumatic/nontraumatic brain injury
nThe vegetative state should not be labeled
permanent until high degree clinical certainty after 12 months post-TBI and 3
months after nontraumatic TBI
Prediction of regaining consciousness
nEtiology
nAge
nBest recovery: children, followed by
adult< 40y/o, �������A��non-traumatic
nAlso poor in severe cerebral
atrophy in CT, and bilateral absence of SSEP potentials
nDuration of unconsciousness
�ثe Unconsciousness duration vs �@�~�����Ӿ��v
|
|
Regain consciousness by one
year |
|
|
Unconscious duration |
TBI |
Non-TBI |
|
One month |
40-50% |
|
|
3 months |
36% |
7% |
|
6 months |
21% |
��C |
Minimally conscious state
nSome evidence of awareness
nVisual tracking and/or motor
behavior that is nonreflexive and contingent on environmental events
Rehabilitation of patients of impaired consciousness
nRemove obstacles to recovery, treat medical
complication, education/ counseling to the family
nR/O artifact of examination techniques,
sedating drug, systemic illness, malnutrition
nDrug-induced sedation can be magnified in
injured brain
nAnit-hypertensive: use clonidine, ACEI,
Ca-blocker, diuretic instead of propranolol, methyldopa, metoprolol
nAmitriptyline can cause paradoxically
sedation of its anticholinergic effect
Rehab. of unconscious patient remains controversial
n Sensory stimulation(coma
stimulation) used multiple modalities: no evidence of effect in chronic
traumatic unconsciousness
n Pharmacological treatment
remains the most promising intervention
Neurostimulant medication
nMethylphenidate,
Dextroamphetamine
nAntiparkinsonian agents
nAmantadine
nBromocriptine
nLevodopa/Carbidopa
nAtypical: TCAs, protriptyline, SSRI,
sertraline, Bupropion, venlafaxine
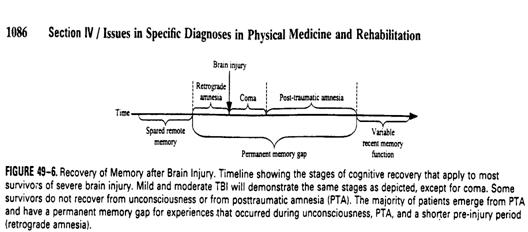
Posttraumatic amnesia (PTA)
nDefinition: the period during which the
patient��s ability to learn new information is minimal or nonexistent
nS/S: Confabulation
nAssessment for end of PTA:
nOrientation to time and place
n
nGOAT: 0 �V 100, >75 defined normal
nEnd of PTA: The date after which GOAT score
> 75 consistently
nPermanent amnesic disorder: failure to
clear from PTA.
nOther nontraumatic brain injury may have
the same condition, and alternative term may be more suitable.

Agitation
nDuring PTA, many patients may have
agitation.
nS/S: emotional lability, motor
overactivity, physical or verbal aggression, poor attention
nTo date, no consensus on definition, some
authors defined it a subtype of delirium.
nAssessment: ABS: Agitated Behavior scale
nEvaluate 3 realms: disinhibition,
aggression, lability
nStudies: minority of TBI patient have
agitation with aggression, many more: only agitation with motor restlessness,
many others: no agitation
Rehabilitation of Posttraumatic Amnesia
nThe rehabilitation unit should
have a system for identifying how closely each patient needs to be supervised
nAvoid overstimulating the patient
with a demanding herapy schedule, unrealistic therapeutic epectations, and
unpleasant emotional interactions with family or staff.
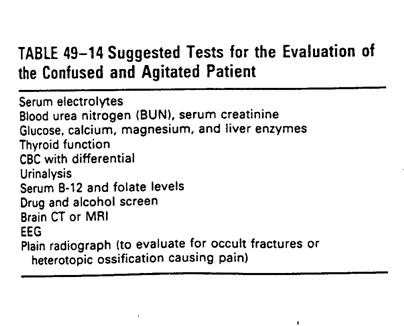
Rehabilitation agitated patient
nRule out medial and neurologic conditions
---> then dx agitation, table 49-14
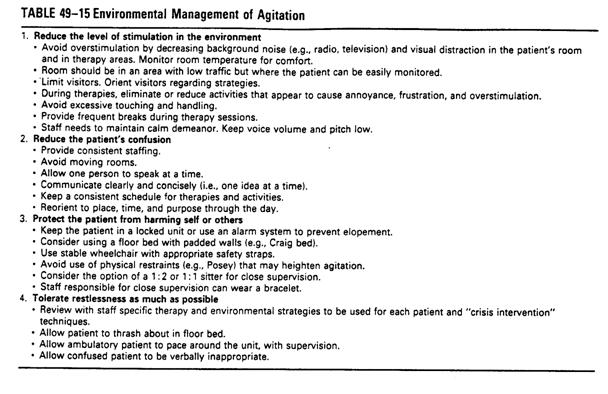
nRestraints: minimal degree if necessary
nFloor bed: Craig Bed (�p��)
nEnvironment management:
nreduce the level of stimulation in the
environment
nreduce the patient��s confusion
nProtect the patient from harming self or
others
nTolerate restlessness as possible
nMedication: when environment management
failed, no guideline exists to date


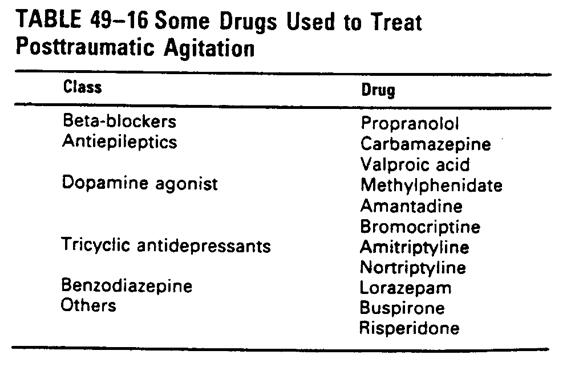

n TBI experts ���֥�haloperidol, benzodiazpines
n Lorazepam(ativan) 1~2 mg IM may
be needed only in uncontrolled emergent
and danger violent behavior patient.
Medical complication of TBI
nSpasticity
nPosttraumatic epilepsy
nPosttraumatic hydrocephalus
nCranial nerve damage
nPosttraumatic hyperthermia
nSleep disorder
nPulmonary complications
nGI and nutritional complications
nThrombophlebitis
nGU complications
nMusculoskeletal complication�X HO
nSexual and reproductive functioning


Spasticity
nCerebral origin spasticity(COS) different
from that of SCI
nGreater extensor tone in extremity, less
spasm
nSpasticity that interferes with functional
goal warrants treatment.(table 49-18)
nRecent approach suggests concurrently
employed various treatment strategies:
nPrevention of nociceptive stimuli, ROM,
stretch, casting, orthosis, modality, invasive motor point block, operation
nProper positioning: ��muscle relaxation, improve alignment, ��primitive reflex; lying supine
can increase the tonic labyrinthine supine reflex(TLSR), thereby increasing
extensor tone


nAntispasticity ball splint for
spastic hand
nChemoneurolysis: phenol, alcohol,
anesthetic agent; side effect: swelling, pain, bleeding, dysesthesia, DVT
nChemodenervation: Botulinum
toxin A, side effect: atrophy, pain, infection; �`�g������phenol block ������
nITB: intrathecal baclofen,
concentration more in lumbar spine à more effective in lower limbs
spasticity, �]����W�Ϫ�spasticity�����O�D�n��indication
n Trial: 50, 75, 100 mg on
consecutive days, if effective, then ITB pump is implanted
nOP: tendon lengthening or
transfer, rhizotomy more performed in children���H����favor
n Oral medication generally not recommended
due to it might interfere cognitive function, especially diazepam and baclofen
nDantrolene sodium may be
medication of choice
n It acts peripherally, at the
muscle, à ��depolarization induced Ca influx
into the sarcoplasmic reticulum(SR)
n Side effect: can also be
sedating, general weakness. Liver enzyme should be monitored
nTizanidine, �\2 agonist, ��presynaptic inhibition of motor neuron, ��release of excitatory amino acids.
Posttraumatic epilepsy (PTE)
nIncidence in hospitalized TBI: 5%
nRisk factors
nPenetration injury
nseizure during the first week (early
seizures)
ndepressed skull fractures
nacute intracranial hematomas
ndural tearing
npresence of foreign bodies
nfocal sign such as aphasia and hemiplegia
nago �� 65 y/o
nloss of consciousness > 1 days
nPTA > 24 hrs
nPrevention: no longer
recommended except penetrating injury or early seizure noted, not beneficial
nDrug of choice:
n Carbamazepine: drug of choice
n Most late seizure are
simple/complex partial seizure, carbamazepine as effective as phenytoin in
control generalized seizure, and more effective in partial seizure
n Side effect: GI distress,
headache, dizziness, diplopia, bone marrow suppression àkeep WBC > 3000 cells/mm3 �䤤neutrophil���� �� 50%
n Initial sedative, but fewer
cognitive side effect than carbamazepine Valproic acid(Depakene)
n Gabapentin(Neurontin)
n Not significant cognitive side
effect, not requiring monitoring blood level
n Duration of anticonvulsant: most
clinician DC medications after 1-2
yrs seizure-free years
Posttraumatic hydrocephalus
nTBI: most are NPH or communicating type
nS/S of normal pressure hydrocephalus(NPH)
nTriad: gait disturbance, mental
deterioration, urinary incontinence
nVentricular enlargement and normal CSF
pressure
nIn severe TBI case, more subtle change may
be related to NPH: functional decline, seizure, emotional problems, abnormal
posturing, increased spasticity, Newly onset of hypertension
CT criteria to define hydrocephalus
n1. ��Distended�� appearance of the anterior
horns of the lateral ventricles
n2. Enlargement of the temporal horns and
third ventricle
n3. Normal or absent sulci
n4. If present, enlargement of the basal
cisterns and 4th ventricle
n* Periventricular lucency was used as an
indicator of communicating hydrocephalus
SPINAL CORD INJURY (SCI)
The earliest reference to spinal cord
injury (SCI) is found in the Edwin Smith Surgical Papyrus, written between 2500
and 3000 B.C., where it is described as "an ailment not to be treated".
Much has changed in spinal cord care over the centuries, particularly in the
last 50 years as it relates to increasing survival, life expectancy, community
reintegration, and quality of life. Major advances include the specialized
spinal cord centers of care, model SCI centers funded by the National Institute
on Disability and Rehabilitation Research (NIDRR) in the United States
Department of Education, establishment and growth of organizations and journals
dedicated to SCI care, and the development of the subspecialty of SCI medicine
in 1998. This subspecialty addresses the prevention, diagnosis, treatment, and
management of traumatic and nontraumatic etiologies of spinal cord dysfunction.
The advances of the last 20 years alone have been dramatic in terms of the
understanding of the pathology of the initial and secondary aspects of the
injury, and the barriers that must be overcome to enhance recovery. Newer
techniques to improve function and intervene at the cellular level for possible
cure are being developed. These will further allow individuals who sustain an
SCI to be more independent in the future.
Selected Tracts
The majority of descending corticospinal
fibers cross at the medulla to become the lateral corticospinal tract(CST). A
small number of CST fibers do not decussate at the medulla and descend via the
anterior CST before crossing at the level of the anterior white commissure.
Although often depicted in many representations of the spinal cord, the
existence of a somatotopic organization of the lateral CST has recently been
challenged.
The ascending dorsal white columns cross
in the medulla, via the medial lemniscus, then go on to the thalamus. The
spinothalamic tracts, which carry pain, temperature, and non-discriminative
tactile sensations, cross obliquely in the ventral white commissure of the
spinal cord, ascending a level as they do so. 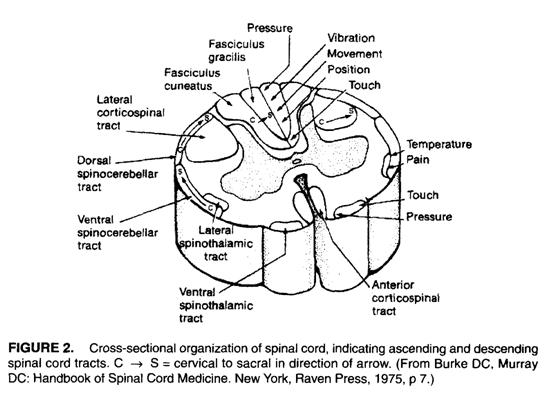
Classification of SCI
1.Perform a sensory exam in 28
dermatomes for pin prick and light touch,
including rectal sensation. The sensory
level is the most caudal
level with intact (grade 2)
sensation.
2. Perform a supine motor exam
of 10 key muscle groups, including
voluntary anal contraction. The
motor level is the most caudal
level with grade ��3, where all muscles rostral to it are grade 5.
3. Determine the neurologic
level, which is the most caudal level
at which both sensory and motor
modalities are intact bilaterally
4. Classify as complete or
incomplete. Completes have no motor or
sensory function, including deep anal
sensation, preserved in
sacral S4-5. (Note: SSEPs may be useful
in differentiating
complete versus incomplete SCI in pts
who are uncooperative or
unconscious. SSEPs are also unaffected
by spinal shock.)
5. Categorize by American Spinal
Injury Association (
Impairment Scale, A-E. (Determine the
rone of partial preserva-
tion ifASIA A.)
|
C2 Occipital
protuberance |
T1 Medialantecubital fossa |
L3
Medialanterior knee |
|
C3
Supraclavicular fossa |
T2 Aper of the anilla |
L4
Medialmalleolur |
|
C4 Top of the
AC joint |
T4 Medial to nipple |
LS
Medialdorsalfm |
|
C5 Lateral
antecubital fossa |
T10 Lateral
to umbilicus |
S1 Inf lal
malleolur |
|
C6 Dorsal
proximal thumb |
T12 lnguinal
ligament |
S2
Poplitealfossa |
|
C7 Dorsal
proximal mid finger |
L1 B/t T12 and L2 |
S3 Ischial
tuberosi* |
|
C8 Dorsal
proximal 5' finger |
L2 Medial anterior thigh |
S4-5
Analmucocutan lu |
|
C5 Elbow flexon |
L2 Hip flexors |
|
C6 Wrist extensors |
L3 Knee extensors |
|
C7 Elbow extensors |
L4 Ankle DF |
|
C8 FDP of 3rd digit |
L5 EHL |
|
T1 Small finger abductors |
S1 Ankle PF |
Sensory are scored as O
(absent). 1(impaired, including hyperaesthesia). 2 (normal), or NT When sconng
pin prick inability to distinguish pin prick from LT is scored 0/2. Muscles are
graded from O (total paralysis) to 5 (normal active movement with FROM against
full resistance), or NT
A Complete No sensory or motor
function is preserved in the sacral segments S4-5. The rone of partial preservation
(only used in
B Incomplete - Sensory but no
motor function is preserved below the neurologic level and includes sacral
segments S4-5.
C Incomplete Motor function is
preserved below the neurologic level, and more than half of the key muscles
below the neurologic level have a muscle grade <3
D Incomplete - Motor function is
preserved below the neurologic level, and at least half of key muscles below
the neurologic level have a muscle grade ��3.
E Normal - Sensory and motor
function are normal.
Note: For an individual to
receive a grade of ASIA C or D, there must be sensory or motor S4-S sparing In
addition, theindividual must have either: )) voluntary anal sphincter
contraction or 2) sparing of motor function >3 levels below the motor level.
Spinal Cord Injury Clinical Syndromes
Anterior cord - These may be due to retropulsed disks/vertebral fragments, aortic
clamping during surgery, or lesions of the anterior spinal artery.
Intraoperative SSEPs (which primarily monitor the posterior column pathways)
may miss the development of an anterior cord syndrome. There is variable loss
of motor and pinprick sensation, with relative preservation of proprioception
and light touch. Prognosis for motor recovny is generally considered poor.
Brown-Sequard - Etiologies may include stab wounds or tumors. Hemisection of the cord
produces ipsilateral weakness,
hyperreflexia, and proprioceptive loss and contralateral loss of pinprick and
temperature sense. The prognosis for ambulation is best among the incomplete
SCI syndromes.
Cauda equina - Cauda equina injuries may be due to neural canal compression or fxs of
the pelvis, sacrum, or spine at L2 or below. The syndrome can be described as
"multiple radiculopathies" since the cauda is comprised of
lumbosacral nerve roots. Sequelae depend on the roots involved and may include
bowel/bladder areflexia, erectile dysfunction, saddle anesthesia, and flaccid
LEx weakness that can progress to complete paraplegia. Radicular pain is common
and can be severe. Anal, bulbocavemosus, plantar, and ankle deep tendon
reflexes may be lost. Recovery is possible because the nerve roots can
regenerate. Consultation for possible early, but not necessarily emergent,
surgery is indicated.
Central cord - This syndrome is typically seen in older persons with cervical
spondylosis following neck hyperextension injury, resulting in upper > lower
limb involvement and sparing of the lowest sacral segments. Bowel, bladder, and
sexual dysfunction is variable. The postulated mechanism of injury involves
cord compression both anteriorly and posteriorly, with inward bulging of the
ligamentum flavum during hyperextension in a stenotic spinal canal. Penrod
retrospectively studied
Conus mednllaris - A pure conus medullaris lesion (e.g.,intramedullary tumor) can result
in saddle anesthesia and bladder anal sphincter/erectile dysfunction due to
cord injury at S2-4. Anal (S2-4), bulbocavemosus (S2-4), and ankle deep tendon
reflels (S1,S2) may be lost if there is injury to the corresponding level of
the spinal cord. Prognosis for recovery is poor.
Conus medullaris lesions due to
trauma (e.g, L1 vertebral body fx) are typically accompanied by injury of some
of the lumbosacral nerve roots, resulting in a variable degree of LEx dysfunction.
Neurological prognosis and functional outcomes
1.
�ƹ�W�Ҧ�traumatic SCI survivor���g���Y�ص{�ת�neurological recovery�A��Y�ǯf�H�o�ث�_�i�ɭPlevel function�ﵽ�C��_�i���{���ˬd�M�q�E�_�[�H�ʴ��C
2.
�b�a��injury zone��segment���g�ǥ\���_�άO��root recovery���n�M�bspinal cord functional global
improvement�Ϥ��X�ӡA�]����̷|�ɭPASIA Impairment Scale�����ܡC�@��Ө��A�f�H�Y�@�}�l�Y���{�X�dz\�B�ʥ\��O�d�A�ӥB���˫���Y�����g�ǫ�_���ܡA�|���̦n���w��C
3.
���X�gpaper��������Mstrength����_�i�F�G�~���[�A�b���˫�@�Ӥ��@�~����motor recovery���t�v�|�b�Y���Ӥ�ֳt�U���C�CIncomplete injury��P't�binjury zone ��_���t�|�֩�complete injury��P't�A���L�̫�_���{�פ����o�|��n�C�X�G�Ҧ��b���˫�@�Ӥ�٤O�u��1/5��2/5��muscle���@�~��|��_��3/5�]antigravity�^�C
4.
���motor function�̫�@�`���U�Ĥ@�`neurological level�A�Ymuscle�u��0/5���٤O�A�f�H�u��1/3�����|��_��3/5�٤O�C���p�b��motor function�̫�@�`�U�G�`muscle power�O0/5���ܡAfunctional recovery�O���֪��C
5.
�btetraplegia�f�H���W�W��muscle weakness���G�ϬM���P�{��upper motor neuron�Mlower motor neuron��damage�C�bvoluntary muscle�@compound muscle action potential���ٹq�Ϥ��R�Χ@�����ٹq��root mean square measure�]����ڴ��q�^�q�`���U�����upper motor neuron��lower motor neuron weakness�C�_���p�e�]�i�ھڳo���G�[�H�ק�C
6.
�Q��ASIA Impairment Scale�M�����e���AFrankel classification�i���ͬ۷��h����ƨӴy�z���˰ϰ�w�ᱡ�ΡCComplete injuries (grade A) ���f�H�����t���w��A�b�p���ˮ`��grade�ٷ|���ܪ����Φ��p�u��0%��9%�C
7.
���MASIA impairment grade A��case���ɱߴ��|�����ܤơA�s���]�A�o�{���\�hcase�@�}�l���ˮɦ�concurrent condition�]��brain injury��sedation�^�ӧ�ê�F���T�������C�qNSCISC model�Ҵ���information�i��grade�ܤƪ����Ρ]Table 55-3�^�A�@�ӶW�L5000�H���Frankle grade���ܪ�study�]��ܦbincomplete injuries���f�H�����Ϊ��w��C
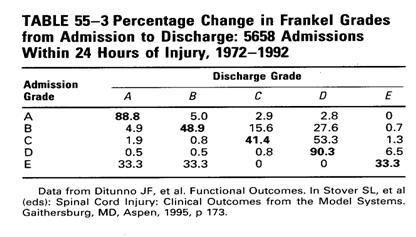
8.
�u��Sensation���O�d���H�b���g�ǤW�|�������{�ת��ﵽ�CPinprick sensation���O�d���G��light tough sensation preservation����j���|��_ambulation��O�A���H�����o�i��O�ǻ�pinprick sensation��spinothalamic tract����a��corticospinal tract���t�G�C
9.
�binjury zone�H�U���즳motor function�O�d���H�]�Acentral cord syndrome�MBrown-Sequard syndrome���f�H�b�\���_�W�|���̦n���w��C
10.
Central cord syndrome���f�H�j�����̲׳��|�o��hand-function�Mcontrol bladder�Mbowel function����O�CAmbulation��_�{�שM���ˮɦ~�֦����A�V�~����_�U�h�CBrown-Sequard��Pt���̦n���w��A�W�L90%��Pt�b���ˤ��Ӥ������_�B�檬�A�C
Expected Functional Levels (I, independent: A, assist: D.
dependent)
C1-3 �V
{
Ventilator-dependent (or may have phrenic nerve pacing);
{
D for secretion management.
{
I with power WC mobility and pressure relief with equipment. otherwise
essentially D for all care (but I for directing care).
C4 �V
{
May be able to breathe w/o a ventilator.
{
May use a mobile arm support for limited ADLs if there is some elbow
flexion and deltoid strength.
C5 �V
{
May require A to clear secretions.
{
May be I for feeding after setup and with adaptive equipment,
n
e.g., a long opponens orthosis with utensil slots and mobile arm support.
{
Requires A for most upper body ADLs.
{
Most pts will be unable to do self clean intermittent catheteriration
(CIC).
{
I with power WC; some users may be I with manual WC on non-carpeted,
level, indoor surfaces.
{
Some may drive specially adapted vans.
C6 �V
{
May use a tenodesis orthosis and short oaponens orthosis with utensil
slots.
{
I with feeding except for cutting food.
{
I for most upper body ADLs after setup and with modifications
n
(e.g., velcro straps on clothing);
{
A to D for most lower body ADLs, Including bowel care.
{
Some males may be I with CIC after setup; females are usually D.
{
Some pts may be I for transfers using a sliding board and heel loops, but
many will require A.
{
May be I with manual WC; but power WCs are also often used, especially for
longer distances.
{
May drive a specially adapted van.
C7 �V
{
Essentially I for most ADLs, often using a short opponens splint and
universal cuff.
{
May require A for some lower body ADLs.
{
Women may have difficulty with CIC.
{
Bowel care may be I with adaptive eguipment, but suppository insertion may
still be difficult.
{
I for mobility at a manual WC level, except for uneven transfers.
{
Pt mav be I with a non-van automobile if the pt can transfer and
load/unload the WC.
C8 - Completely I with ADLs and mobility
using manual WC and car.
Paraplegia
- Trunk
stability improves with lower lesions.
Upper and mid thoracics may stand and
ambulate with b/l KAFOs and Lofstrand crutches (i.e., swing-through or swing-to
gait), but the intent is usually exercise, not mobility.
Using orthotics and gait assistive devices,
lower thoracics and L1 SCI pts can do household ambulation and may be I
community ambulators.
L2-S5 SCI pts may be community ambulators
with or w/o orthotics (i.e., KAFOs or AFOs) and/or gait assistive devices.
(AFOs generally compensate for the ankle weakness, while canes and crutches
primarily compensate for hip abduction and extension weakness.)
Selected Issues in SCI
Autonomic dysrenexia (AD) �V
It can occur in 48-85% of pts
with SCI at T6 or above'. (Since resting SBPs can be 90
Long-term routine urinary tract surveillance after SCI �V
Upper tract f/u can include
renal scan with GFR or renal scan with 24hr Cr clearance qyr to follow renal
function. US can be done qyr to detect hydronephrosis and nephrolithiasis.
Lower tract f/u can include urodynamics (UDS) once the bladder starts exhibiting
uninhibited contractions (or at around 3-6mos post-injury), then as determined
by the clinician (often done qyr or q2yr). Routine cystoscopy to potentially
diagnose neoplasm at an earlier rather than later stage should be performed qyr
after l0yrs of chronic indwelling (urethral or suprapubic) catheter use or
sooner (atier 5yrs) if there are additional risk factors (heavy smoker,
age>40, h/o many UTls).
Posttraumatic syringomyelia �V
It is seen in -3-8% of
posttraumatic SCI pts as neurologic decline, or up to 20% on autopsy. It can
develop as early as 2mos to yrs post-SCl. Pain is often worsened by coughing or
straining, but not by lying supine. Ascending sensory loss, progressive weakness
(including bulbar muscles), �� sweating, orthostasis, and Homer's
syndrome may also be seen. Dx is by MRI. Tx is usually observational and
symptomatic. Surgical interventions are available for large, progressive
lesions.
Sexual function, fertility �V
Females:
44-55% of women with SCI can achieve orgasm. Menses typically returns w/in 6mos
post-SCI, and reproductive function is preserved. Incidence of prematurity and
small-for-date infants is high, but there is no increase in spontaneous
abortions. Spinal anesthesia is recommended during delivery for pts with SCI at
T6 or above.
Males:
With complete SCI, reflexogenic erections can usually be achieved, although
ejaculation is rare. With incomplete SCI, reflexogenic erections are usually
attainable; ejaculation is less rare than for completes; and some pts can
achieve psychogenic erections. Complete or incomplete injuries below T11 may
result in erections of poor quality and duration. Infertility is common after
SCI, due to factors including retrograde ejaculation and poor sperm quantity
and motility. E[ectrovibration for ejaculation (the ventral penile shaft is
stimulated) requires that the pt be >6mos post-injury and have L2-S1 intact.
aecrroejaculation (seminal vesicle and prostatic stimulation through the rectum)
is another option.
Tendon transfer surgery �V
Triceps fUnction can be restored
in the C5,6 SCI pt with a posterior deltoid-to-triceps or a biceps-to-triceps
transfer. Lateral key grip can be restored in a C6 SCI pt via the modified
Moberg procedure, which involves attachment of the brachioradialis (C5,6) to
the flexor pollicis longus (C8,T1) and stabilization of the thumb CMC and IP
joints.
Electrodiagnosis
Purposes of Electrodiagnosis
•
Localization
•
Guidance for disease
severity
•
Characterizing of disease
evolution and estimate of prognosis
•
D/D neuropathy and
myopathy
•
General evaluation in
diseases of neurons, roots, plexus,
peripheral nerves, NM junctions, and muscles
Possible complication and side effect
•
May interfere normal pacemaker function
•
May cause infection when patient with lymph
edema
•
May cause continuous bleeding if patient with
hemophilia or under heparin/warfarin treatment
•
May cause arrhythmia with artery or central
venous line
•
May cause pneumothorax
Clinical Assessment
•
History
•
Physical examination
•
Differential
diagnosis
•
Initial plan
History
•
Time course
•
Quality
•
Distribution
•
Medical history
Systemic disease�GDM, alcohol
consumption, rheumatic disease
Medication�Gdisease, toxic
exposure, anticoagulant
•
Family history
Physical
examination
•
Muscle strength
•
Sensation
Pinprick, light touch
Two-point discrimination
Vibration
•
Muscle stretch reflex
Biceps(C5), brachioradialis(C6), triceps and pronator teres(C6, C7)
Knee(L4), ankle(S1), medial hamstring or tibialis posterior(L5)
Hoffmann��s sign and Babinski��s sign
•
Provocative test
Phalen��s test�Gmedian nerve
entrapment at the wrist
Tinel��s sign�Gnon specific
Spurling��s sign�Gcervical
radiculopathy
Straight leg raising test or other sciatic nerve stretch maneuver�Glumbosacral
radiculopathy
Differential
iagnosis and initial plan
•
For focal or distal problems,
like entrapment neuropathy, NCS first.
•
For proximal lesion,
or diffuse/multifocal process, (like radiculopathy), EMG first.
Needle Electomyography
•
Preparing the patient
•
Deciding on an
electrode to use
•
Steps of the needle
EMG examination
•
False-positive and
false negative findings on needle EMG
Preparing
the patient
•
Explanation:
Experience
Risk and benefits
•
Consent for high-risk
procedure�Ae.g. intercostal muscle EMG
•
Positioning
•
Room temperature
Deciding
on an electrode to use
¬ Surface electrode:
Disposable, self adherence, size
¬ Needle electrode
Monopolar
•
Stainless steel coated with Teflon with bare metal tip
•
Separate reference and grounding
Concentric
•
Hollow stainless steel with a central platinum or nichrome-silver wire
surrounded by epoxy
resin
•
Cannula serves as reference, only separate grounding needed
Single-fiber electrode
Monopolar vs. Concentric electrode
|
|
Monopolar |
Concentric |
|
Recording
territory |
wider |
smaller |
|
Reference |
distant:
noiser |
closer:
quieter |
|
Discomfort
|
fewer
(Teflon coating) |
more |
|
Recording
potential |
larger |
smaller,
with possibly fewer phases |
|
Duration
|
Same |
same |
|
Clinical
use |
broad
area recording, e.g. finding the FP or PSW |
restricted
area recording, e.g. quantitative motor unit analysis |
|
Cost |
cheaper |
expensive |
Steps
of the needle EMG examination
•
Insertional activity
•
Spontaneous activity
•
Motor unit analysis
•
Recruitment
False-positive
and negative findings on needle EMG
•
False-positive�Goverreading of subtle changes:
as insertional activity,
polyphasic waves,
recruitment,
end-plate potential �� fibrillation
potential
•
False-negative
Too short temporal course ( < 2~3 weeks)
Too few muscles examined or each muscle insufficient examined
Nerve
Conduction Studies
•
Measurement of compound or sensory nerve action
potentials
•
Measurement of compound muscle action potentials
•
Late responses
Measurement of compound or
sensory nerve action potential�Gconduction velocity
•
Speed (conduction velocity or latency)�G
Traditionally use
peak latency
Onset latency
CV = d/t or d/(t
�V activation time);
CV = distance
difference/onset latency difference; with two point
stimulation
•
Factors affecting CV
Physiological�Gcold limbs,
aging, increased height
Pathological�Gdemyelination or
loss of the fasting conducting fiber
Measurement of compound or
sensory nerve action potential�Gamplitude
•
Measurement�G
Baseline to peak
Peak to peak
•
Factors affecting amplitude�G
Physiological�Gdistance between nerve and electrode, aging, cold (increase
amplitude)
Pathological�Gany axonal loss
lesion distal to dorsal root ganglion
Measurement of compound muscle
action potentials
•
Recording over muscle�G
N-M junction�G~1 msec
Conduction along
muscle fiber�G~ 3
Use onset latency
•
Amplitude�Gpeak to peak, baseline to peak
•
Intact SNAP amplitude with ��CMAP amplitude
Motor neuron
disease or other intraspinal process
Severe
radiculopathy
Selective motor axonopathies�]heavy-metal neuropathy, porphyria, some
demyelinating neuropathy�^
N-M junction
disease
Primary myopathy
Late response�G
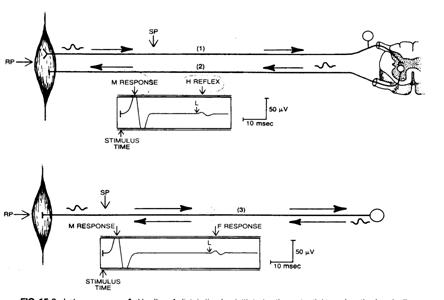

Late response�GF-wave
•
Occurs when a small percentage (3-5%) of
antidromically activated motor cell discharges.
Supramaximal
stimulation
•
Assessment of multifocal or diffuse processes,
especially affecting proximal area.
Demyelinating
polyneuropathy
Late response�GH-reflex
•
Most easily elicited in soleus, with submaximal
stimulation
Flexor carpi
radialis, foot intrinsic, quadriceps
Children < 3
y/o
•
Stimulation
Long-duration
pulses
Relaxation or
minimal plantar flexion
Stimulation rate
0.2 Hz
Clinical use of H-reflex
•
Latency depends on age and leg length
•
Abnormal finding�G
Side to side
difference of latency: 1.2 msec
Side to side
difference of amplitude: 40%
F-wave V.S. H-reflex:
|
|
F-wave |
H-reflex |
|
Stimulation
|
Supramaximal
|
Submaximal |
|
Amplitude
|
Small,
(3-5% of M-wave) |
Large
|
|
Consistency |
Unstable
|
Stable
|
|
Mechanism
|
Antidromic
activation |
Monosynaptic
reflex |
Interpretation
•
Normal verse abnormal
•
Principle of localization
•
Deducing the pathophysiology from the
electrophysiological results
•
Timing of electrophysiological changes
•
Estimating prognosis
Normal versus abnormal
•
Nerve conduction study and quantitative EMG
results are not always conclusive normal or abnormal
•
Reference value: Mean with 2-SD, one-sided,
based on normal Gaussian distribution
•
Diagnosis are best made when multiple
��abnormalities�� are demonstrated in a pattern consistent with clinical
presentation.
Principles of localization
•
EMG
For the very
proximal lesion
Based on finding
abnormalities distal to a branch point, with normal findings proximal to that
point
Intraneural
topography makes some trouble�G
•
Ulnar nerve lesion over elbow
•
Sciatic nerve lesion over hip
•
Common peroneal nerve lesion
•
Nerve conduction study
Based on
demyelination
•
Focal slowing
•
Conduction block: focal drop of amplitude
Difficulty when axon
loss with little demyelination
Deducing the pathophysiology
from the electrophysiological results
•
Neuropraxia or focal conduction block
Large amplitude
drop when stimulation proximal to the lesion compared to distal stimulation
•
Demyelination
Slowing of
conduction
•
Axon loss
EMG�Gmore sensitive
NCS�Gsmall
amplitude of CMAP and SNAP; quantify the degree of axon
loss and prognosis
Timing of electrophysiological
changes
•
Day 1 after an axon loss lesion�G
EMG�Greduced
recruitment
NCS�Gdrop amplitude
when stimulation proximal to the lesion site
•
Day 7 to 10 (CMAP: 7 day, SNAP 10 or 11 day)�G
NCS�Ga conduction
block lesion can be distinguished from axon loss
•
Days 14 to 21�G
EMG�Gstarts to show
Fib/PSW
•
Fib/PSW means denervation, not mean whether it
is ��active�� or
��ongoing�� axonal loss
•
Radiculopathy�Gparaspinal muscle: 10~14 day, distal muscle 3~4
weeks
•
Fibrillation amplitude�G2 months�G600 uV�A6 months�G300 uV�Aone year�G
100 uV
Estimating prognosis
•
EMG and CMAP amplitude
•
Factors�G
Pathophysiology
problems�G
•
Focal slowing
•
Conduction block: recovery in weeks to months
•
Axontemesis
•
Neurotemesis�GEDX can��t find out the supporting tissue injury
Time since
onset
Distance between
lesion and target muscle
•
Critical window for target muscle�G18~24 months
Pediatric rehabilitation
Primary reflex
|
Reflex |
Stimulus |
Response |
Age of Suppression |
|
Moro Startle |
Sudden
neck extension Sudden noise, clapping |
Shoulder
abduction, shoulder, elbow, and finger extension, followed by arm flexion
adduction |
4-6 months |
|
Rooting |
Stroking
limbs or around mouth |
Moving mouth, head toward
stimulus, in search of nipple |
4 months |
|
Positive supporting |
Light
pressure or weight bearing on plantar surface |
Legs extend for partial
support of body weight |
3-5 months Replaced
by volitional weight bearing with support |
|
Palmar grasp |
Touch
or pressure on palm or stretching finger flexors |
Flexion of all fingers, hand
fisting |
5-6 months |
|
Asymmetric tonic neck |
Head
turning to side |
Extremities extend on face
side, flex on occiput side |
6-7 months |
|
Symmetric tonic neck |
Neck flexion Neck extension |
Arms flex, legs extend Arms extend, legs flex |
6-7 months |
|
Reflex |
Stimulus |
Response |
Age of Suppression |
|
Plantar grasp |
Pressure
on sole distal to metatarsal heads |
Flexion of all toes |
12-24 months when walking is
achieved |
|
Autonomic neonatal walking |
On
vertical support plantar contact and passive tilting of body forward and side
to side |
Alternating
autonomic steps with support |
3-4 months |
|
Placement or placing |
Tactile contact on dorsum of
foot or hand |
Extremity flexion to place
hand or foot over an obstacle |
Before end of 1st year |
|
Neck righting or body
derotational |
Neck rotation in supine |
Sequential body rotation from shoulder to pelvis toward direction of face |
4 months Replaced
by volitional rolling |
|
Tonic labyrinthine |
Head
position in space, strongest at 45 degree from horizontal Supine Prone |
Predominant extensor tone Predominant flexor tone |
4-6 months |
Some critical milestones to remember by age:
|
Age (mo) |
Milestones |
|
|||
|
1 |
Lift head (prone), vocalizes |
|
|||
|
3 |
Follows, laughs, smiles, has
good head control |
|
|||
|
5 |
Plays with feet, reaches for
and grasps objects |
|
|||
|
6 |
Sits with support |
|
|||
|
8 |
Sits without support |
|
|||
|
9 |
Plays peekaboo (���߿�), gets to sitting position; parachute reflex present; stranger anxiety |
|
|||
|
10 |
Pulls to stand, cruises,
babbles(�����ǻy) |
|
|||
|
12-14 |
First words; walks |
|
|||
|
18 |
Multiple single words; uses
spoon, removes clothes |
|
|||
|
24 |
Uses two-word phrases, throws
overhead; ��terrible twos�� |
|
|||
|
30 |
Knows full name, puts on
clothing |
|
|||
|
36 |
Jumps, pedals tricycle, learns
nursery rhythms |
|
|||
|
48 |
Hops, plays with others |
|
|||
|
Mobility |
Fine motor |
Language |
Social skill |
||
|
Rolling
(first prone to supine, then reversed)�X4-5 months Sitting independently�X6-7
months Walking�X1 year Runs�X2 years Stairs
(adult style)�X4 years Skips�X5
years (boys later than girls) |
Grasping items�X4-5 months Hand-to-hand transfer�X6 months Pincer�X10-11 months Feed
with spoon�X18 months Scribble(�õe)�X18 months Copies
circle�X3 years Copies
cross�X4 years Copies triangle�X5 years |
Babbling�X7-8 months Single
words�X1 year Body
parts�X18 months Short sentences�X2 years Full sentences�X3 years Paragraphs�X4 years Knows
color�X5 years |
Interactive
game (pat-a-cake)�X9months Take
off clothes�X15 months Copies
housekeeping�X18 months Parallel play�X3 years Social interaction�X4 years |
||
|
Verbal score |
Adult and Older Child |
Young Child |
|
5 |
Oriented |
Smiles,
oriented to sound, follows objects, interacts |
|
4 |
Confused, disordered |
Cries
but consolable, interacts inappropriately |
|
3 |
Inappropriate words |
Cries
but is inconsistently consolable, moaning (��|) |
|
2 |
Incomprehensive sounds |
Inconsolable crying, irritable |
|
1 |
No response |
No response |
Scoring of eye opening and motor
responses same as for adults
The characteristics and clinical
associations of the following gaits:
Spastic, crouched, hemiparetic, waddling, and
ataxic gaits
|
Gait |
Characteristics |
Clinical Association |
|
Spastic |
Adducted hips Internal rotation of hips Toe walking |
Cerebral palsy |
|
Crouched |
Weak quadriceps Weak hip extensors Excessive dorsiflexion Hip or knee contractures |
Neuromuscular disease Cerebral palsy |
|
Hemiparetic |
Circumduction of hip Posturing of upper extremity Inversion of foot |
Cerebral palsy Cerebral vascular accident |
|
Waddling (Trendelenburg) |
Weakness of hip girdle |
Neuromuscular disease |
|
Ataxic |
Wide-based gait Coordination problems Poor tandem walking |
Cerebellar ataxia Friedreich��s ataxia |
The definition, risk
factors and clinical effects for cerebral palsy.
- Definition:
- Collection of diverse syndromes
characterized by disorders of movement and posture
- Cause by a non-progressive
injury to the immature brain.

- Diplegia: refers to spastic paresis in
the lower extremities more than in the upper extremities
- Quadriplegia: involves abnormalities
of both upper and lower extremities (typically worse in legs).
- Hemiplegia: abnormalities that involve
an arm more than the ipsilateral leg
- Triplegia: abnormalities that involve
both legs and one arm.
Risk Factors for Cerebral
Palsy
|
Prenatal |
Perinatal |
Postnatal |
|
Congenital malformations Socioeconomic factors Intrauterine infections Teratogenic agents Maternal mental retardation Maternal seizures Maternal hyperthyroidism Placental complications Additional trauma Multiple gestation |
Prematurity (<32wk) Birth weight < Growth retardation Abnormal presentations Intracranial hemorrhage Trauma Infection Bradycardia and hypoxia Seizures Hyperbilirubinemia |
Trauma Infection Intracranial hemorrhage Coagulopathies |
Clinical Effects of Cerebral Palsy
- 1. Disorder of neuromuscular control
- 2. Abnormalities of muscle tone
- 3. Persistent primitive reflexes
- 4. Weakness in individual muscles
- 5. Disordered kinesthetic (��ı��) sense
- 6. Changes in joint alignment
- 7. Bony deformity: hip, spine and feet
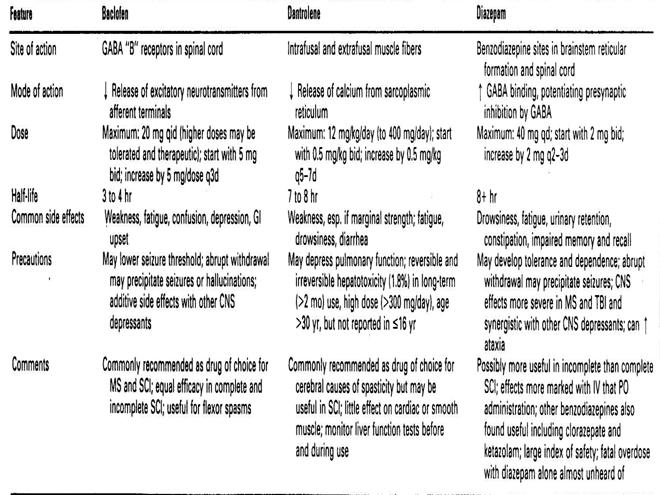
The antispastic medications used in children

What are the indications for Phenol or Botulinum
toxin therapy in cerebral palsy children? What are the advantages and
disadvantages of these two blocking agents.
Indications for Phenol or Botulinum Toxin Therapy
- Spasticity interfering with activities
of daily living and functional activities.
- Spasticity unresponsive to
conservative measures
- Spasticity requiring impractical or
impossible frequency and duration of adjunctive measures such as ROM
therapy
- Spasticity involving antagonist
musculature that interferes with agonist function
- Spasticity that may compromise the
success of planned surgery. Some patients have undergone more than one
surgical procedure at a given joint because the underlying problem of
spasticity was inadequately managed.
Botox


|
Blocking agent |
Botulinum type A toxin |
Phenol block |
|
Administered |
Injected into muscle |
Injected into motor points of
involved muscle |
|
Effectiveness |
Lasts 12 to 30 weeks |
Lasts 4 to 12 months |
|
Advantages |
Easy to administer Diffuses readily into muscle Painless Can be administered without
anesthesia |
Use is widely approved Lasts longer than botulinum
toxin Cumulative effects often occur |
|
Drawbacks |
Effects are always transient Lasts only 12 to 30 weeks Limited approval |
Can be painful May require general anesthesia
during administration Takes more skill to administer |
|
Complications |
No significant complications
reported |
Transient dysesthesias and
numbness Hematomas may occur, which
negate the effects of treatment If a large intravascular
injection occurs, phenol can cause systemic effects such as muscle tremors
and convulsions, as well as depressed cardiac activity, blood pressure, and
respiration |
SDR(SPR): Selective Dorsal (Posterior) Rhizotomy
- Selective dorsal rhizotomy is a
surgical procedure performed to reduce leg joints stiffness and spasticity
in children who have cerebral palsy.
- SPR involves selecting a variable
percentage of sensory nerve rootlets after L2-S1 laminectomy.
- Each sensory nerve root is divided
into 3-5 rootlets. Each rootlet is tested with EMG, which records
electrical patterns in muscles. The severely abnormal rootlets are
cut.
- This results in a decrease in
peripheral excitatory influences on the anterior horn cell in patient with
spastic cerebral palsy.
- L5 and S1 are the most frequently
abnormal roots.
- The most common unwanted post-surgical
effects: hypotonia (usually transitory) , weakness, sensory changes,
bladder dysfunction, and hip subluxation/dislocation have also been
reported.
SDR(SPR): Selective Dorsal (Posterior)
Rhizotomy: Favorable Selection Criteria
��Pure spasticity (limited
dystonia/athetosis)
��Function limited primarily by spasticity
��Not significantly affected by primitive
reflexes/movement patterns
��Absence of profound underlying weakness
��Selective motor control
��Some degree of spontaneous forward
locomotion
��Adequate truncal balance/righting response
��Spastic diplegia
��History of prematurity
��Age 3-8 years
��Minimal joint contractures or spine
deformity
��Adequate cognitive ability to participate
in therapy
��No significant motivational/behavioral
problems
��Supportive and interactive family
Describe the classification of Congenital BPI
Congenital Brachial
Plexopathy(1)
|
Plexopathy |
Level (Nerve Roots/Trunk) |
Clinical Findings (Related
Weakness) |
|
Duchenne-Erb��s palsy (most
common) |
C5-C6��C7 Upper trunk |
Weakness of shoulder
abduction, external rotation, elbow flexion, supination, wrist extension,
finger extension, waiters tip posture. |
|
Klumpke��s plexopathy |
C8-T1 Lower trunk |
Weakness of elbow extesion,
pronation, wrist flexion, hand weakness, associated with Horner��s syndrome |
|
Pan plexopathy |
C5-T1 Arm, entire plexus |
Weakness of all motions listed
above, flail chest |
- Usually unilateral, can be bilateral.
- Most common etiologies: large baby and
shoulder dystocia.
- Caused by injury to brachial plexus by
excessive lateral traction to fetal head or neck and/or intrauterine
propulsion due to force of contraction.
- Treatment: ROM exercise,
strengthening, splinting, encourage use of affected arm.
- Complete recovery occurs in more than
65%. Mild and moderate deficits are seen in 10% each. 15% will have severe
residual deficits.
- The optimum age for surgery is younger
than 9 months because nerve growth factor is at a maximum before 1 year of age, and there is
less nerve scarring and distal muscle atrophy at that age.
- Surgical procedures: neurolysis of
scars, end to end anastomosis with microsurgical fascicular repair and
cable graft of nerve rupture.
1. �o�i��w(developmental delay)�ൣ���w�q?
���ൣ�]���U�ؤ��P����]�A�b�\��o�i�����W�Ӧ~�֩��������{�סA�Ҧp�B�ʡB�P�x��ı�B�y���B�{���B���|�A���B�����Φ欰�U�譱����O�A�Ϩ�b�o�i�W�y���U�ص{�פ��P������{�H(���F���`�Ĥl80%) �A�٤����o�i��w�ൣ�C
��1992 Colorado Interagency
Coordination Council defines that the child exhibit a 20 % delay in
functioning when compared to his or her same age peers.�\��C��P�~�֨ൣ���ʤ����G�Q
��1987 Peterson defines
criterion that a score 2 or more standard deviation below the mean of a
reference group.�C���Ȩ�өΨ�ӥH�W���зǮt
�o�i��w���ŧP�w
����۷�����w�G�q�����Х��F�����ȤU5�зǮt
����ۭ���w�G�q�����Х��F�����ȤU4~5�зǮt
����ۤ���w�G�q�����Х��F�����ȤU3~4�зǮt
����ۻ���w�G�q�����Х��F�����ȤU2~3�зǮt
�����L��w�G�q�����Х��F�����ȤU1~2�зǮt
�åp�o�i��w�ൣ�\��ʶE�_����
���{���o�i��w
���y���o�i��w
���ʧ@�o�i��w
�����|�����o�i��w
�������ʵo�i��w
���D�S�w�ʵo�i��w
Prevalence of Developmental Delay
6-8 % by WHO study
�o�i��w�ध�o�i��������
w²���ൣ�o�i�q�� �]�åp�^
w����ൣ�欰�o�i�q�� �]CCDI�^
w�������X�o�i���� �]�Ш|���^
wBayley Scales of Infant Development (Bayley
II)
w
w
w
wStanford-Binet Intelligence Scale (IV)
wWechsler Intelligence Scale
a. Preschool and Primary Scale (WPPSI-R)
b. Children (WISC-III)
wVisual Motor Integration (VMI)
wStycar Test (Visual Acuity)
wSensory Integration Evaluation
�o�i����
��A.�s�ͨ����O(Neonatal Assessment)
��B.�z�����O(Screening Test)
��C.�E�_���O(Diagnostic tool)
��D. ���J���O (Early Intervention)
A �s�ͨ�������(Neonatal Assessment)
��Apgar Score
��Clinical Assessment of
Gestational Age in the Newborn Infant
��Neonatal Behavioral Assessment
Scale
��Assessment of Preterm Infant's
Behavior
��Neurological Assessment of the
Fullterm & Preterm Newborn Infant
��Morgan Neonatal Neurobehavioral
exam.
��Movement Assessment of Infants
��Milani-Comparetti Motor Development
Screening Test
B �z�������� (Screening Test)
- ����o�i�z�˶q��II
(Revised
- ������֫e�ൣ�o�i�q��
(Chinese Child Developmental
Inventory, CCDI)
- ²���ൣ�o�i�q��
(Simple and Accurate Child
Development Screening Test)
- �̰Ǿ��֫e���q
(Miller Assessment for
Preschooler, MAP)
- �������X�o�i����z���D��
(Infant and Child Comprehensive
Developmental Battery, screening test )
Chinese Child Developmental
Inventory( CCDI)
���֫e���بൣ�o�i�q��
��Gross Motor �ʰʧ@
��Fine Motor ��Ӱʧ@
��Expressive Language �y�����F
��Comprehension Conceptual �y���F��
��Situation Comprehension ���Ҳz��
��Self Help �ۧڷ��U
��Personal Social ���|����
²���ൣ�o�i�q��
����Ӱʧ@
���ʰʧ@
���y�����q
������B�z���|��
D �E�_���o�i����
(Diagnostic tool)
�����ܤ�����o�i�q��
(Bayley Scale of Infant Development,
BSID)
���ʰʧ@�\����q��
(Gross Motor Function
Measure, GMFM)
�����ڴ��ڴ����ʧ@�q��
(Bruininks-Oseretsky Test of
Motor Proficiency, BOTMP)
���������X�o�i����E�_�D��
(Infant and Child
Comprehensive Developmental
���ൣ����ê�����q��
(Pediatric Evaluation of
Disability Inventory, PEDI)
���֤ڭ}�ʧ@�o�i�q��
(
E ���J���o�i�����u��
���������J�q��
(Early Intervention Developmental
Profile, EIDP)
��Portage �����Ш|���ɤ�U
(
���ൣ�V�m���n
���ͬ��A����O�ˮ֤�U
�������״��٪̥\��ʱоǺ��n
��צ����Φ�����H?
�������|
�����S���ݨD���ൣ���Ѧ����o�{�B�����E�_�Φ����v���A�ߧY�����M�~�ʪ������B�_���B�Ш|�B�श�κ֧Q����U�A�H���ĭ��C���ê�{�סA�R���}�ݨ�o�i��O�C
���ൣ����(0-6 ��)
���Q�Φh�M�~��X�ʪA��
����U�����B�a�x���|���������D
����ֵ֨o�g�A�}�o�Ĥl����O
����X�J���|�ζ����A���Ī���֪������|����
�٦׳n��´�W���i²��
��ζW���i�E�_�t�ΡA�W�O�Q�ζW���i������q���S�ʡA�b�Ǽ������|���A�ǥѪi�M�H���´���椬�@�ΡA�p�٦שM�תն��n�����t���A��q�o�ͤϮg�B���g�Χl�������z�{���A�]�Ӥ����X�����´���������ΡA���ѶE�_�W�������C�W���i�ѩ�㦳real time�S�ʡA�i���ˬd�̦b���ˬd���ɭԡA���ʺA�ˬd�A�P�ɥ[�W�̪��W�v(7.5 MHz�H�W)�����Y���X�{�M�m��Doppler�v�����o�i�A��ϱo�W���i�����Φb�E�_�n��´���f�ܤ譱�����@���Q���C�ڭ̹��n��´�A�q�ֽ��착�f�������A���i�H�ϥζW���i�ӧ@�E�_�C���f�٦W���i�A�L�h�b����M�Ȭw�a�Ϩϥαo����֡A�D�n��]�O�j�a�ߺD�Q��MRI�ӧ@�E�_�C����n��´�c�y�A�W���i�i�H��������MRI���E�_�\��A���O���������o����MRI�C�A�ӥB�ާ@��K���t�C�]�ӡA�ڭ̬۫H���n��´���f�ܡA�W���i���ϥι�b�ȱo���s�C
�b��ǶE�_�W�A�ϥα��Y�o�g�@�C��q�W���i�n���i�J�H���´�ɡA�]�������´���n���ܮt���A�y���n��q�����Ϯg�δ��g�A�Φ����P���^�i�T��(echo)�A�o�Ǧ^�i�T���ѱ����t�α�����j�B�ոm�A�A�Q�Τ��P����ܤ覡���{�X�ӡC��ǤW�`�Ϊ���ܤ覡���GA�Ҧ��]���T�Ҧ��AAmplitude
Mode�^�AB�Ҧ��]�G�Ҧ��ABrightness
Mode�^�AM�Ҧ��]�B�ʼҦ��AMotion Mode�^�PD�Ҧ��]���R�ǼҦ��ADoppler Mode�^�A�b���f�٦רt�γ̱`�ϥΪ��h�OB�Ҧ��PD�Ҧ��C�H�U²���W���i�ˬd�b���٦רt�εo�i���{���G
1.
�٦ץ~�ˡG�� �׳̱`�����f�ܴN�O�~�ˡA�Ѩ�O���|�Ϫ��٦סC�H�ۮɥN���i�B�A�H�����B�ʪ��ߦn�W�[�A�ϱo�B�ʶˮ`�ܦ���v�̤�`�Ҹg�`�n�B�z�����D�C���ڭ̦b�B�z�o �@�����f�H���ɭԡA�Y����o��@�Ӧ٦ת������v���A���ڭ̶E�_���T�w�O�۷������U���C�p�G�ڭ̭n�ˬd���٦O�ݩ����L�h���٦סA�ϥΰ��W�v (10 MHz) linear array���W���i���Y�i�H�o����Ϊ��ѹ��סC�Ӧp�G�n�ˬd���٦O�ݩ����`�h���A����N�n�ϥ�5 MHz�A�Ϊ̬O3.5 MHz�� ���Y�~����������z�O�C�٦ת��ˬd�����]�A�𮧩M���Y���٦ת��A�A�ӥB���ݦP���ˬd�٦ת��a�V�P��V�������ϡC�p�G�f�H����������N�A�ڭ̤]�i�H�b�ֽ��� �������W�O���A������N�ɩw�줧�ΡC�̪�ѩ�m��W���i���o�F�A���٦��ت���y���Χڭ̥i�H�[�H��T�������A���٦ˮ`���E�_��O�h�F�@���Z���C�b�@ �ӫ�ʴ��٦פ��X�媺�f�H�A�@�몺B-mode�W���i�ä��Ӯe���q�W���i���^������
(echogenicity) �Ӱ��E�_�A���O���˪��٦P���`���٦פ���A�|����n�W�[�����ΡA���o�����f�H�X�Ѥ���A���Ъ��ˬd�A�i�H�o�{����echogenicity�|���ܤơC�@�Ӿ��T����� (clotted
hematoma)�A�b�W���i�ˬd�e�{���O�@�ӧC�j�����㹳(hypoechoic)�A�ݰ_�Ӧ��ɭԹ��O�G�骺�ˤl�A���O���ɭY�h�w���������|���ѡC
2.
�n��´���~�F�G���n��´���~�F���E�_�A���q���A��xeroradiography�PCT���㹳�O���t�A�@��j�a���O���w�ϥ�MRI�C�i�OMRI�� �������Q�A���ɭ��ٷ|�Q���O���ִ�O�ΡA�]�ӶW���i�b���n��´�~�F���E�_�W�N�����@�Ӭ۷����n���a��C�b���n��´�~�F���W���i�ˬd�ɡA���M�ڭ̬O�ھڸ~ �F������ӿ���ڭ̩Ҩϥα��Y���W�v�����C�C�~�F���E�_�ܤ֭n����ӥH�W�������A���ɭԸ~�F�Ӥj��ݷ|�W�L�A�ҨϥΪ����Y�C�M�ӥثe�w���s�����ذݥ@�A�i �H�N���Y���ʮɪ��v���s���b�@�_�A���������Y���ק����topography�C�ӥB�Q�αm��W���i�A�ڭ̤]�i�H�Ӭ�s�~�F����ު��S�ʡA��p�Q��spectrum analysis�i�H��PI(Pulsative index)�PRI(Resistive index)�����w�C�ثe�w���\�h�����i���X�A�p�G�n��´�~�F�ح�����ޤ���״I�A�ר�b�a�����a��A����c���ܪ����|�N������A�p�GRI�C��0.6�]�O�N���c���ܪ����|������C���M�p�G�O�o�{�@���n�~(cyst)�A����ڭ̤]�i�H�b�W���i�����ޤ��U��������F�ƦܬO��ߪ��~�F�]�i�H�b�W���i�����ޤ��U�A�ΰw�Y����ӭM���ӭM��(cytology)���E�_�A�i�H�K�h�}�M������(biopsy)���B�J�A�����Ȥ���K�y�A�f�H�Ҩ����W�h�]����֡C�ڭ̤��ȥi�H�q�W���i��echogenicity�ӶE�_�ӭM�������A�ӥB�]�i�H�P�_�~�F���Y�O�_���t�ƩάO����A�άO�w�Ū��o�͡A�t�~�]�i�H�d�ݰ��f���ֽ�h(cortex)�O�_������i�ΡC�q�`�ڭ̦b���n��´�ӭM�ˬd���ɭԭn�`�N�^���U�C�X�Ӱ��D�G1.�O�_�u�����@�Ӹ~�F�H2.�~�F���κA�O����H3.�~�F����m�b���̡H4.�~�F����n���h�j�H5.����´�O�_���Ҫi�ΡH���M�p�G�n��´�~�F�O�@���n�~�A�άO����A�άO���F�A�άO�P�����M��(well-marginated)�A��y����C���F�A�ڭ̸����ⴤ�h�P�_���O�}�ʩʽ誺�ɭԡA�N�i�H���������J�ʪ���N�v���C�p�G�٬O���c���ܪ��i��A����MRI�٬O�������AMRI���c�ʸ~�F������(staging)�O�ȱo�ѦҪ��C
3.
�����`�G�������`�n��´���f�ܡA�ר�Orotator cuff�}�����E�_�A�L�h�`�ϥ�contrast arthrography�A���O���èS����k�ӰϤ������γ������}���CMRI�̪�]�Q�j�q�ϥΨӶE�_rotator cuff���f�ܡA���O�P�˹������Χ����}�����f�w�èS����k���Ī��ӥ[�H�P�O�C�����`�ϥζW���i�ӶE�_�A�i��O���٦רt�Τ��ϥγ̦����@�ӳ���C�b7.5-mhz�H�W�����W�v���Y�ϥΤ��U�A�ڭ̹��C��rotator cuff���i�H�M���a�ݨ�A�]�A��W��(supraspinatus)�B��U��(infraspinatus)�A�ӤU��(subscapularis)�A�ФG�Y�٪����Y(long head of biceps)�����C�p�G���@�Ӧ��g�窺�ˬd�̨ӻ��A���rotator cuff�ˮ`���E�_�A�W���i���E�_�v�w�g�i�H�F��ʤ����E�Q�H�W�A�ꤺ�b�o�譱���_�B�]�O�̦��A�\�h��Ǥ��ߪ���v�w�g�ֿn�F�۷����g��C
4.
�b���`�G�W���i�b�b���`�e�f���E�_�A�D�n�O�Φb������A�ר�b���ऻ�Ӥ�H�e�A�ѩ�Ѱ��Y���|���t�ơA���q��X���L�k��ܡA���@�ǥ��ѩ��b���`��ݪ��f�w�A�H���E�_�W�����n��arthrography�Ϊ̬OMRI�A�e�̨㦳�I�J�ʦӥB���ݱ�����g�u���Ӯg�A��MRI���E�_�h�۷��O�ɡB�O���ӥB�]������ڡC�W���i�b�o�譱���E�_���ѤF�@�Ӧw���S����g�u�A���h���t�S���T���E�_�C�ثe�H�۶E�_�N���i�B�A��power Doppler��i�H���T���E�_�X�Ѱ��Y���ح���y�����ΡA�i�H���U������v�M�w�b�����ѩ��b���`���(CDH or DDH)�_��H��]�ۻI�����סA�H�K�]�����L�j�v�T�Ѱ��Y���ʦ���a��(avascular necrosis)�����͡C�t�~���p�Ĥl�`�����b���`�n���g�άO���w�g�A�]�i�H�ܥ��T���Q�ζW���i�Ӱ��@�E�_����¦�A�A�[�W�W���i�ˬd�����ޤ��U�A�i�H²��B���t�B���T��������b���`�ح����n���Τ��w�Ӱ����E�_�Ϊv�����̾ڡC
5.
�����`�G�W���i�Ω����`���E�_�A�ثe���T�w���O����ƽ��n��(synovitis)�A�����a(collateral ligament)���~�˩Mpatellar tendon���E�_�C�ثe�]���H���i����Q�r���a���f�ܡA�W���i�w�g�i�H���T���E�_�X�ӡC�ӹ��e�Q�r���a�Υb��n���A�ثe�٦b�o�i�����q�C
6.
���ﳡ�G���ﳡ���W���i�E�_�A�ثe���Z�̦n���O�������H��٦ٸx(posterior tibial tendon)�譱���E�_�C�L�h����H��٦ٸx�ɱ`���F�{���ˬd�_���~�A�b�v���Ǫ��ˬd�n�atenography�Ϊ�MRI�C���p�G�ϥζW���i���ˬd�A���Ȩ��t�B�K�y²��A�ӥB���T�C�ٸx���ܤƥ]�Atenosyonvitis�M�����_���A�W���i���i�H�۷����T���E�_�X�ӡF�t�~�A����ˡA�Q�ζW���i�ڭ̤]�i�H�ܪ������[���~�����a�A�]�Aanterior talofibular ligament�Mcalcaneofibular ligament�������T�v���A�@�������E�_����¦�C
7.
��L�G����ީί��g���f�ܡA�ثe�W���i�]�����������Z�C��pcarpal tunnel syndrome���E�_�A�Q�ζW���i�]�i�H�۷����T���E�_�X�ӡC��ޤ譱���ˬd�A�ר�bcolor Doppler���ݥ@�H�ӡA�X�G�i�H���o��angiography�@�ˡA�ӥB�����Ѧ��I�J�ʡC�ثe�ѩ�power Doppler
image���o�i�A�ϱo�C�y�t����ޥ��}�n���㹳�A�b�n��´����perfusion�譱���q���ܱo��ǻP���T�C
�`���ӻ��A�W���i�㦳�۷��h���n�B�G1.�����㦳��g�ʡA������߯f�H�����ˬd�|�����g�ʪ��ˮ`�C2.�����㦳�I�J�ʡA�ˬd���ɭԯf�H���|�h�W�C3.��������t�B��K�A�������Ԯɶ��C4.������K�y�A����MRI������Q�C5.���i�H���U�ڭ̦b�W���i���Y�����ޤ��U�A���@�DzG�骺����άO�ӭM�Ǥ譱������A�H�ѶE�_���ΡC6.�i�H�H�۾ާ@�̪��ާ@�A�i�H�N���g�B��ޡB�ٸx���A���κc�y����´���@�a�V���v���l�ܡA����MRI�q �`�u�O���_�����X�ڭ̩ҭn�ݨ쪺�v���C��X�H�W�o�Ǧn�B�A�W���i�b���f�٦רt�Ϊ����ΡA���B�_����άO���g����v�ӻ��A���ܦ��E�_�W���������ȡC�ר��H�ۮɥN���i�B�A�w�餣�_����}�A�ڭ̤]�۫H�b���[���N�ӡA�W���i�@�w�|����n���^�m���Ѥj�a�E�v���W�@�Ӭ۷��n���E�_�����C