| ¡@ |
Polycystic Ovary Syndrome (PCOD)
The polycystic ovary syndromeAround one in five women have polycystic ovaries. This term describes the appearance of the ovaries when they are seen on an ultrasound scan. The polycystic ovary syndrome (PCOS) is the name given to a condition in which women with polycystic ovaries have one or more additional symptoms. So not all women with polycystic ovaries have polycystic ovary syndrome, but all women with PCOS do have polycystic ovaries.
The diagnosis of PCOS has been made much easier in recent years by the availability of ultrasound scanning. Before this, only women with the most severe symptoms could be diagnosed with accuracy; today, the condition can be detected even when the problems are only mild.
Normal ovaries
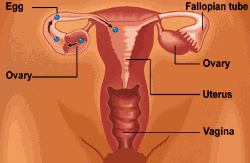
Women have two ovaries; they are located in the pelvis alongside the uterus (womb). Their main functions are to release eggs and produce hormones.

At birth, the ovaries are provided with thousands of eggs, each surrounded by cells which develop into a small, fluid-filled blister known as a follicle. Each month, in women with regular periods who are ovulating normally, one of these follicles will grow to about 20 millimetres in diameter and then release an egg (ovulation), which passes into the fallopian tubes. Here, fertilization takes place, before the fertilized egg (embryo) continues to the uterus to implant in the lining (endometrium) and develop as a pregnancy. If no egg fertilizes, the endometrium is shed as a menstrual period around 14 days after ovulation.
Three important groups of hormones - estrogens, androgens and progesterone - are also produced in the ovary. These in turn are regulated by the release of two further hormones from the pituitary gland at the base of the brain - follicle stimulating hormone (FSH) and luteinizing hormone (LH). These two 'reproductive' hormones influence the development of the follicle and the timing of ovulation.
Polycystic ovaries
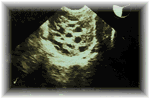
Polycystic ovaries contain many small cysts - at least ten. Some of these cysts contain eggs, some are dormant, and others might secrete hormones. The cysts are quite small, usually no bigger than 8 millimetres, but they are clear enough on ultrasound to allow an accurate diagnosis. Blood tests might also reveal changes in hormone levels which are characteristic of polycystic ovaries, but these levels vary considerably from one woman to another.
 |
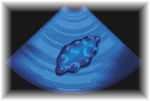 |
| Transvaginal ultrasound scan of a polycystic ovary | |
Transvaginal ultrasound scan of a polycystic ovary
Doctors are still not entirely clear why some women have polycystic ovaries. There may be a hereditary link, and they are present in women of all ages, many of whom show no symptoms of PCOS. In other words, ovaries do not suddenly become polycystic; but women who have always had polycystic ovaries may develop symptoms at any time.
The cause of polycystic ovaries is believed to involve an inability of the ovaries to produce hormones in the correct proportions. The pituitary gland senses that the ovary is not working properly and in turn releases abnormal amounts of LH and FSH - which may mean that unusually high levels of LH are circulating in the bloodstream.
The symptoms of PCOS
1.Menstrual irregularities
The imbalance of hormone production from both the ovaries and pituitary may
result in either irregular ovulation or no ovulation at all (known as 'anovulation').
Menstrual periods may therefore become irregular - perhaps heavier than
usual, perhaps occurring after long gaps (oligomenorrhoea) or perhaps not at
all (amenorrhoea). Some women notice pelvic pain, which may be related to
the effect of hormones on the flow of blood through the pelvic veins.
2.Fertility
Irregular ovulation usually means that pregnancy is more difficult to
achieve; similarly, if ovulation is not taking place, it is just not
possible to conceive without treatment. So women with irregular cycles
hoping to get pregnant will have a better chance once their monthly cycles
have returned to normal.
3.Miscarriage
While miscarriage seems an unfortunate chance event for most couples, it is
now known that women with PCOS who have high circulating levels of LH may be
at greater risk. The explanation is probably that too high a level of LH in
the bloodstream makes it more difficult for the egg to develop within the
follicle, and for an embryo to implant within the uterus.
4.Skin problems
One of the hormones which may be released in unusually high amounts from the
ovary is testosterone, the 'male' sex hormone which circulates in both men
and women. Excessive testosterone levels in women may be a cause of acne on
the face and back, or unwanted hair on the face, chest, arms and legs. The
levels of testosterone in women with PCOS are still much lower than those
found in men.
 The everyday problems of PCOS are often only amenable to medical treatment,
but there are also some lifestyle changes which can improve symptoms. For
example, doctors know that the body's hormone balance can be upset by
excessive body weight, and certainly PCOS is more common in obese women than
in those with a correct weight-to-height ratio. Equally, some women with
polycystic ovaries only develop symptoms when they put on weight. So a
correct weight-to-height ratio will help, and this can be measured by an
equation called 'the body mass index' (BMI). Your BMI is your weight in
kilograms divided by your height in metres and then squared - or as doctors
would write, kg/m2. A normal BMI is between 20 and 25.
The everyday problems of PCOS are often only amenable to medical treatment,
but there are also some lifestyle changes which can improve symptoms. For
example, doctors know that the body's hormone balance can be upset by
excessive body weight, and certainly PCOS is more common in obese women than
in those with a correct weight-to-height ratio. Equally, some women with
polycystic ovaries only develop symptoms when they put on weight. So a
correct weight-to-height ratio will help, and this can be measured by an
equation called 'the body mass index' (BMI). Your BMI is your weight in
kilograms divided by your height in metres and then squared - or as doctors
would write, kg/m2. A normal BMI is between 20 and 25.
A danger to health?
The small cysts detected in polycystic ovaries do not get any bigger; in fact, they usually disappear only to be replaced by other similar cysts. They remain small (no bigger than 8 millimetres) and do not require removal by surgery. Sometimes, larger cysts (over 20 millimetres) might release an egg. Only very large cysts (over 50 millimetres) require surgery, and they can occur in any woman, whether she has polycystic ovaries or not. There is no link between the cysts of polycystic ovaries and ovarian cancer. However, although the risk is still very rare, women with few or absent periods are at an increased risk of endometrial cancer. This can happen when the womb lining (endometrium) becomes too thick; regular shedding in the form of a period prevents this. If the endometrium appears thick or irregular on an ultrasound scan, a D&C (dilatation and curretage) operation might be advised.
Obese women with polycystic ovaries are also at greater risk of heart disease - simply because excess weight is linked to high blood pressure and excessive levels of cholesterol in the bloodstream, both known risk factors for heart disease. A high fibre, low fat and low sugar diet at a young age may help reduce these risks in later life - as will stopping smoking. Diabetes in later life, in which the body becomes unable to use sugar efficiently, is also associated with excess weight. Medication might be needed, but weight loss and a lower intake of carbohydrates will also help.
Indeed, being overweight is probably the cause of the greatest and most frequent problems for women with PCOS.
Management of PCOS
It thus goes without saying that all women with polycystic ovaries should try to maintain a normal weight and to have regular periods. Medical treatment is usually confined to those with the troublesome symptoms of PCOS.
1.Menstrual irregularities
Irregular periods are a nuisance - as well as a suggestion of some hormonal
disorder or risk of endometrial thickening. For women who have no wish to
become pregnant, the contraceptive pill offers the easiest solution. This
will produce a regular (though artificial) cycle and regular withdrawal
bleeding during the Pill-free week. Today, most gynaecologists would
recommend a low-dose variety for women with polycystic ovaries. Women who
cannot take the Pill might find improvement from a progestogen-only
treatment, usually taken for 12 days every one to three months. This will
induce bleeding, without any of the side effects associated with the
estrogen in the Pill.
Any irregular bleeding while you are taking the Pill should be checked by a doctor. A D&C or ultrasound scan might be thought advisable; similarly, a routine cervical smear should be taken once every three years.
2.Difficulty in conceiving
While failure to ovulate is the usual reason for infertility in women with
polycystic ovaries, it is also worthwhile ensuring that other important
factors - like your own fallopian tubes or your partner's semen - are also
OK.
* Monitoring ovulation. In normal cycles, ovulation takes place 14 days before a period starts - so only if your cycle is 28 days will ovulation take place on day 14. If your cycle is 27 days, for example, ovulation will be on day 13; if it is 35 days, ovulation will be on day 21. These sums are important to get right if you are timing sexual intercourse to coincide with ovulation. The most reliable way to predict ovulation is with an over-the-counter urinary test kit. This measures the surge of LH which occurs around the time of ovulation. Testing should begin a day or so before you expect to ovulate, while intercourse should take place on the day the test shows a colour change, and on the day after. Temperature charts can indicate hormonal changes in the cycle, but are not accurate predictors.
Ovulation can be monitored by ultrasound, but this of course requires visits to hospital and so is usually reserved for women having more complicated treatments and those who have difficulties with the urine tests.
A blood test seven days after presumed ovulation allows doctors to measure the level of the hormone progesterone to check if ovulation did in fact take place.
 Of course, most of this monitoring - whether simple or more complicated - is
performed so that sexual intercourse might be timed to coincide with
ovulation. If you have regular cycles and are ovulating normally,
intercourse two or three times a week should achieve a supply of sperm
sufficient to fertilize the egg when it is released. Long periods of
abstinence appear to worsen sperm function. Many patients report that
intensive monitoring can remove much of the spontaneity from their sex
lives. A short break from treatment - perhaps a month or two - might relieve
the pressure and allow more relaxed love-making.
Of course, most of this monitoring - whether simple or more complicated - is
performed so that sexual intercourse might be timed to coincide with
ovulation. If you have regular cycles and are ovulating normally,
intercourse two or three times a week should achieve a supply of sperm
sufficient to fertilize the egg when it is released. Long periods of
abstinence appear to worsen sperm function. Many patients report that
intensive monitoring can remove much of the spontaneity from their sex
lives. A short break from treatment - perhaps a month or two - might relieve
the pressure and allow more relaxed love-making.
* Drugs to induce ovulation. In cases where ovulation is irregular or non-existent, drugs can be used. The most common is clomiphene citrate, which is taken as a tablet for five days from the second day of menstruation. Results show that four out of five women given clomiphene do ovulate, but only about one in three actually become pregnant. The starting dose is usually 50 milligrams, which may be increased to 100 milligrams. Clomiphene can cause thickening of mucus in the cervix, so a post-coital test can tell doctors how well the sperms are surviving in the genital tract.
However, whilst clomiphene is a useful drug for many women with infertility problems, it is not always suitable for those with PCOS because it can cause an exaggerated rise in blood levels of LH which could impede fertilization or increase the chance of miscarriage. So, if clomiphene has not been successful in women with PCOS within six months, more investigations and alternative treatments are usually called for.
Side effects with clomiphene have been reported, notably stomach and bowel upsets, hot flushes, bloating, headache, dizziness, depression and breast discomfort. Multiple pregnancy is a risk whenever ovulation is induced with fertility drugs - in natural conception the risk is about one in eighty, in induced ovulation about one in twenty. There is no increased risk of birth defects from fertility drugs.
* More complicated treatments. If tablets fail, injectable hormones stimulate the ovaries more directly. The most common injections involve a group of hormones known as gonadotropins which are derived from human urine. Human menopausal gonadotropin (hMG) contains both FSH and LH activities, while 'purified' FSH preparations contain only tiny amounts of LH. Recently, gonadotropin preparations have been produced synthetically by means of modern biotechnological methods. These new preparations can be given by subcutaneous (under the skin) injections rather than by the deeper intramuscular injection required with the original preparations. These products work adequately in women with PCOS. However, because the polycystic ovary contains many small cysts (which are, in effect, follicles), the ovary is very sensitive to stimulation by these hormones. In view of this, courses of treatment begin with low doses, and the growth of the follicles is carefully monitored by ultrasound. These scans may be supported by measurements of estrogen release from the ovary into the bloodstream. If monitoring shows that too many follicles are developing and the risk of multiple pregnancy is high, doctors will usually suspend treatment and cancel that cycle.
A second gonadotropin - human chorionic gonadotropin (hCG) - is given to stimulate release of the egg from the follicle. This preparation is given when the ovary contains one or more mature follicles. hCG takes between 36 and 48 hours to work - so if it is given in the morning, ovulation can be expected during the following evening and night.
Women with PCOS given gonadotropins are at an increased risk of a rare but dangerous condition known as ovarian hyperstimulation syndrome - so careful monitoring is essential. The condition occurs if many follicles are stimulated and results in abdominal distension and nausea.
* Laparoscopic ovarian diathermy. A new, minimally-invasive operation which is performed through a laparoscope looks set to replace the more traumatic 'wedge resection' in which a part of the ovary was removed surgically. The new procedure - known as laparoscopic ovarian diathermy - actually burns parts of the ovary to correct any hormonal abnormalities and thus make ovulation possible.
 * In vitro fertilization (IVF). IVF, the 'test-tube baby' technique
in which a woman's eggs are fertilized with her partner's sperm in the
laboratory, is usually recommended to women who have blocked fallopian
tubes, or men with poor sperm. IVF is sometimes offered to women with PCOS
who want to conceive when other treatments have failed. However, PCOS on its
own is not an indication for IVF. Women with PCOS who do undertake IVF are
at greater risk of the ovarian hyperstimulation syndrome and must be
carefully monitored.
* In vitro fertilization (IVF). IVF, the 'test-tube baby' technique
in which a woman's eggs are fertilized with her partner's sperm in the
laboratory, is usually recommended to women who have blocked fallopian
tubes, or men with poor sperm. IVF is sometimes offered to women with PCOS
who want to conceive when other treatments have failed. However, PCOS on its
own is not an indication for IVF. Women with PCOS who do undertake IVF are
at greater risk of the ovarian hyperstimulation syndrome and must be
carefully monitored.
3.Miscarriage
Miscarriage in women with PCOS is thought to be associated with raised
levels of LH. However, it has not been shown to be preferable to suppress
the release of LH before inducing ovulation - which can be done by using a
drug known as gonadotropin releasing hormone analogue (GnRHa). GnRH
analogues can be given as a daily injection or as a nasal spray. Once blood
levels of LH have fallen, ovulation can be induced with either hMG or FSH.
Laparoscopic ovarian diathermy also results in a lowering of LH levels in
the bloodstream and might also help reduce the risk of miscarriage.
4.Skin problems
The usual therapies for acne and unwanted hair are a combination of estrogen
(as found in the contraceptive pill) and an 'anti-androgen' hormone like
cyproterone acetate. The cyproterone is taken for the first ten days of the
cycle, and the Pill for the first 21 days. This therapy, of course, has a
contraceptive effect - and so is of little use to those trying to conceive.
There are alternative treatments without any contraceptive effect, so this
important issue should be discussed with your doctor.
Waxing and electrolysis can be helpful, especially while waiting the
several months for hormonal treatments to work. However, they should only be
performed by trained therapists, as scarring can result from unskilled
treatment. If PCOS is diagnosed as the cause of the problem, correction of
the hormonal abnormality is the logical solution.
PCOD is a
common condition that presents in two forms: a) Classical PCO (Stein
Leventhal Syndrome) with: 1.Obesity b) Non Classical PCO or PCO
variants. This subtle condition is far
more prevalent than the classical form of the
disease and patients present with some of the
features (1-6 above) but not all. Specifically
these patients may be thin but still manifest
aspects of classical PCO. Patients with PCO have a
disruption to several hormonal systems leading to
an abnormal ovulation, hirsutism, and possibly
insulin resistance. a) Pituitary -There is an
excess production of LH compared to FSH (greater
than 3: 1) leading to disruption of the
menstrual cycle and increased androgen (male
hormone) production in the ovary. a) History and physical
examination (this will enable classical PCO
patients to be differentiated from the non
classical. Treatment of PCO can take
many forms including: Illustration of
Polycystic Ovary
Table 1 *Consensus reached
by the National Institutes of
Health/National Institute of Child Health
and Human Development The exact diagnostic criteria
of PCOS have not been clearly elucidated,
despite a National Institutes of
Health/National Institute of Child Health and
Human Development conference held to reach a
consensus on this disorder. Nevertheless, the
conference participants concluded that PCOS
should be defined by the following criteria
for research, which are listed in order of
importance: hyperandrogenism and/or
hyperandrogenemia; oligo-ovulation; exclusion
of other known disorders, such as Cushing¡¦s
syndrome, hyperprolactinemia, or congenital
adrenal hyperplasia, and, possibly, polycystic
ovaries on ultrasound.28
Other laboratory findings and clinical
manifestations may present which can prove
helpful in making a diagnosis. Laboratory Findings:
2. Hirsutism (excess hair growth) with elevated
male hormone levels ( i.e. testosterone).
3. Irregular or absent menstruation since
puberty.
4. Lack of ovulation and infertility.
5. Ovaries with many small cysts, hence the term
polycystic.
6. Insulin resistance with a greater risk of
developing diabetes.![]()
b) Ovary-Increased production of estrogen
without progesterone may lead to the development
of a thickened uterine lining (endometrial
hyperplasia) and possibly uterine cancer over
many years. Testosterone production is also
increased in the small cysts of the ovary and
this may be converted to more estrogen in the
fat cells.
c) Adrenal-An elevation in adrenal androgens (DHEAS)
is seen in some PCO patients.
d) Insulin Resistance- This phenomenon relates
to an insensitivity of the PCO patient to
insulin requiring the body to produce a greater
amount of this hormone to process a given amount
of carbohydrate. Since insulin has the effect of
increasing testosterone production in the ovary,
a self-perpetuating cycle is produced.![]()
There are several aspects important to the
diagnosis of PCO:
b) Hormonal testing including fasting glucose
and insulin levels.
c) Ultrasound to visualize the ovaries.
d) Endometrial biopsy to exclude pre cancerous
uterine conditions.
b) Regulation of menses may be accomplished
with regular administration of progesterone or
the use of an appropriate oral contraceptive
(one low in androgens).
c) Ovulation induction with:
¡@
2. Gonadotropins such as Follistim or
Repronex with the occasional use of a GnRH
agonist (Lupron)or antagonist (Antagon).
Close monitoring to prevent hyperstimulation
of the ovary is mandatory.
3. Dexamethasone may be used to suppress
adrenal androgen production.
¡@
e. Hirsutism- May be treated with an
appropriate oral contraceptive preparation,
Spironolactone, and cosmetic approaches such
as electrolysis and laser.
f. Surgery- In refractory cases, laparoscopic
surgery with a YAG laser may be used to reduce
the ovarian production of testosterone.
Polycystic ovary syndrome (PCOS), formerly
known as the Stein-Leventhal syndrome, was
first described by Drs. Stein and Leventhal in
1935.1 It is
one of the most common endocrinopathies
affecting women of reproductive age.2,3
Most women who develop PCOS have
oligomenorrhea or amenorrhea, hirsutism,
obesity, and infertility.4
Another common finding in women with PCOS is a
normal onset of menarche that is ¡§frequently
followed by painless, erratic menses.¡¨3
PCOS is a familial disease in some cases.5
The syndrome is characterized by
endocrinologic and reproductive abnormalities.3,6
The prevalence of PCOS has been estimated to
be between 2%¡V20%, depending on its
definition.7
Utilizing the diagnostic criteria of ovulatory
dysfunction, clinical hyperandrogenism (i.e.,
hirsutism) and/or hyperandrogenemia in women
without hyperprolactinemia, congenital adrenal
hyperplasia, or Cushing¡¦s syndrome, one group
of investigators estimated the prevalence to
be approximately 4.6%. Knochenhauer and
colleagues evaluated 369 women, of whom 148
blacks and 129 whites consented to
participate.7
However, most epidemiological studies exclude
women of diverse ethnic backgrounds and only
include those who have visited their
healthcare practitioner with complaints
consistent with this condition.
The general therapeutic approaches to this
disorder range from the use of medications
(e.g., antiandrogens, oral contraceptives)
that treat the clinical manifestations, to
operative techniques (e.g., ovarian wedge
resection and diathermy) to manage the
physiological abnormalities, to weight
reduction that restores ovulation.8
Pathogenesis
Various studies have found positive
correlations between insulin and androgen
levels.9,10
Dunaif et al.9
demonstrated a significant positive
relationship between unbound testosterone and
insulin in obese women, which is similar to
results from other studies in obese women with
PCOS.11,12
Investigators have shown that in vitro theca
cell growth and production of androgen are
stimulated by their binding of insulin and
insulin-like growth factor 1 (IGF-1).13,14
In obese women with PCOS, diazoxide
administration for 10 days significantly
lowered bound and unbound testosterone serum
concentrations; this was mediated by decreased
insulin plasma concentrations.15
Further support is given to the hypothesis of
insulin-induced hyperandrogenemia from studies
demonstrating that gonadotropin releasing
hormone (GnRH) agonist suppression of
testosterone and androstenedione does not
affect insulin serum concentrations or
insulin-stimulated glucose disposal.16,17
It appears that insulin resistance is not
mediated by androgen plasma concentrations. In
fact, most evidence supports the hypothesis
that hyperinsulinemia causes increased ovarian
androgen production, which leads to
hyperandrogenemia in women with PCOS.16,18-21
Jarrett and coworkers22
studied the binding affinity of insulin to
human ovarian membranes that contained
granulosa and thecal cells, ovarian stroma,
and connective tissue and concluded that there
is ¡§significant, high-affinity binding of
insulin to human ovarian tissue.¡¨ Poretsky et
al. found that insulin receptors are present
in the human ovarian tissue that is composed
primarily of stroma.23
Ovarian stroma produces various steroids in
vitro: androstenedione, dihydrotestosterone (DHT),
estradiol, estrone, progesterone, and
testosterone.24
Another hypothesis for the etiologic basis of
PCOS, put forth by Rosenfield and colleagues,
involves cytochrome p450c17alpha.25
An enzyme with dual functions, p450c17alpha
converts progesterone to
17alpha-hydroxyprogesterone and
17alpha-hydroxyprogesterone to androstenedione
through its 17alpha-hydroxylase and
17,20-lyase activity, respectively. Another
enzyme, 17beta-reductase, converts
androstenedione to testosterone.26
Rosenfield and colleagues proposed that the
abnormal enzymatic activity of cytochrome
p450c17alpha leads to increased production of
androgens within ovarian thecal cells. The
hyperfunctioning of this enzyme may be due to
excessive stimulation of thecal cells by
luteinizing hormone (LH) or due to the thecal
cells¡¦ inability to be desensitized by LH.25
Nestler and Jakubowicz reported that decreased
insulin levels may indeed reduce p450c17alpha
activity, either directly or indirectly, by
reducing LH serum concentrations.26
Pathophysiology
As the name implies, PCOS may be suspected
based on the appearance of polycystic ovaries
on ultrasound. However, this finding is not
specific for PCOS, for polycystic ovaries are
found in women who have hypothalamic
amenorrhea and congenital adrenal hyperplasia.27
In fact, many patients with PCOS do not have
polycystic ovaries, and some women who ovulate
normally and lack the characteristics that
satisfy PCOS criteria may have
ultrasound-identified polycystic ovaries.27,28
A remarkable finding in women with polycystic
ovaries is the greater-than-normal number of
graafian follicles and the lack of mature or
preovulatory follicles.4
This may reflect the early luteinization of
follicles.4
The biochemical profile and histological
findings do not correlate in women with PCOS,27
nor does the ovarian appearance correlate with
symptomatology.27
Diagnosis
PCOS Diagnostic Criteria for Research
Purposes*
Listed from
most to least important:
Source: Based on reference 28
¡@
|
Table 2 |
|
Serum
Concentration Elevations • Testosterone (bound and unbound) • Androstenedione • Estrone • Luteinizing hormone (LH) • Insulin • Glucose • Prolactin • Possibly, dehydroepiandrosterone (DHEAS) Below-normal Plasma Concentrations • Follicle-stimulating hormone (FSH) • Sex hormone-binding globulin (SHBG) • Possibly, dehydroepiandrosterone (DHEAS) |
The pattern of gonadotropin (LH
and follicle-stimulating hormone, FSH)
secretion in women with PCOS differs from the
normal menstrual cycle. The normal cyclic
secretion of FSH and LH is absent.29
There is an abnormal elevation in LH9,10,29
in relation to consistently low levels of FSH
throughout the cycle,29
such that the LH/FSH ratio is greater in women
with PCOS.10,12,19,30
The elevated serum concentrations of LH
ultimately result in increased production of
estrogen, which has a negative feedback on FSH
release.31,32
When LH levels are elevated in women with PCOS,
they approach the concentrations achieved
during the normal midcycle LH surge and are
greater than those normally found in the
follicular phase of the menstrual cycle.31,32
Elevated serum androgen concentrations,
specifically testosterone and androstenedione,
have been demonstrated in women with this
disorder,9,12,18,19,30,33
and the source of the excess androgens are the
ovaries.4,27
Barbieri and colleagues conducted a controlled
in vitro study of the effect of insulin
release on ovarian stroma in women with
hyperandrogenism. Their results demonstrated
an increased secretion of androgens from
ovarian stroma.18
Other investigators34
tested the fluid from small ovarian cysts in
women with PCOS and found a high concentration
of androstenedione and no detectable
estradiol-17beta or estrone. Cyst fluid
containing a high amount of androstenedione
and small amounts of estradiol and estrone is
characteristic of polycystic ovary syndrome.34
Higher serum concentrations of androstenedione12,35
and of total and unbound testosterone (the
biologically active moiety of testosterone
that is not bound to sex hormone-binding
globulin) were demonstrated in women with PCOS
as compared to controls.9,30,33,36,37
Some investigators19,36
have found estrogen levels to be similar in
women with and without PCOS. However, Lobo and
colleagues discovered that women with PCOS had
higher levels of unbound estradiol-17beta.36
Also, higher-than-normal estrone serum
concentrations appear to be a ¡§usual abnormal
finding¡¨ in women with PCOS.9,30,33,37
The elevation in estrone levels may be the
result of androstenedione aromatization.30,35,38
The elevated levels of unbound estrone,30
testosterone, and estradiol38,39
are due to a reduction in sex hormone-binding
globulin (SHBG) capacity.30,39
The increase in unbound estradiol may be a
causative factor of inappropriate gonadotropin
secretion in PCOS.39
Lower serum concentrations of SHBG in the PCOS
group than in the control group has been
reported.30
Additionally, obese women with PCOS have
significantly lower androstenedione plasma
concentrations compared to nonobese women with
PCOS. Obesity increases the aromatization of
androstenedione and, therefore, the
manufacture of estrone.30,33,38
Obesity is also associated with reduced SHBG
plasma concentrations.30,38
The normal estradiol to estrone ratios are
reversed in women with PCOS such that there is
a greater amount of estrone than estradiol.19
In some reports, dehydroepiandrosterone
sulfate (DHEAS) serum levels were not as
significantly elevated in controls as in those
with PCOS.10,30,36,37
However, other studies demonstrated that DHEAS
levels are lower in women with PCOS than in
those without PCOS because DHEAS is secreted
almost exclusively from the adrenal cortex.
Therefore, increased androgen production by
ovaries will lead to reduced androgen
production from the adrenals, as signified by
DHEAS. Hyperinsulinemia is also associated
with lower serum concentrations of DHEAS.
Patients with PCOS have higher basal serum
insulin concentrations than normal women.10,12,19
Women with PCOS also tend to have a greater
insulin response to the oral glucose tolerance
test than those without PCOS.19
Women with PCOS have reduced
insulin-stimulated glucose utilization37
and require higher amounts of insulin to
stimulate glucose disposal as compared to
controls.33
It is important to note that the insulin
resistance happens in the face of normal
glucose tolerance in nonobese women with this
disorder; therefore, insulin resistance is not
dependent on glucose tolerance19,33,37
or obesity.9,10,12,19,33,37
However, obese women with PCOS have a greater
degree of hyperinsulinemia than nonobese women
with PCOS30 and obese normal women.30,40
Also, the serum concentration of C-peptide is
higher in obese women with PCOS than in normal
obese women.40
Insulin resistance in obese patients with
polycystic ovary syndrome is an important
determining factor of hyperinsulinemia.40
Higher-than-normal fasting glucose serum
concentrations37
and more impaired glucose tolerance tests9
occur in obese women with PCOS versus obese
women without this condition. The elevated
plasma glucose concentrations appear to be
secondary to insulin resistance in the
periphery and in the liver,11,37
since insulin is required for glucose uptake
in the hepatic and peripheral tissue.41
Although its occurrence is uncommon, hyper-prolactinemia
exhibited as galactorrhea may be a presenting
feature of PCOS.3,27,37
In summary, the abnormal laboratory findings
that may be detected in women with PCOS are
serum concentration elevations of testosterone
(bound and unbound), androstenedione, estrone,
LH, insulin, glucose, prolactin, and possibly
DHEAS. Below normal plasma concentrations of
FSH, SHBG, and possibly DHEAS may also be
measured.
Clinical Manifestations:
Common clinical manifestations of PCOS are
listed in TABLE 3. Obesity is
not an uncommon finding.4
An estimated 50% of women with PCOS are
obese¡Xi.e., have a body mass index (BMI)
greater that 27 kg/m2.42
Those with obesity and PCOS increase their
risk
of developing impaired glucose tolerance,
whereas nonobese women with PCOS (and
therefore insulin resistance) have in most
cases, normal glucose tolerance.9
Obesity is associated with a reduced amount of
insulin receptors, as well.12
|
Table 3 |
|
| Condition | Significance |
| Obesity | Occurs in 50% of patients; associated with increased risk of impaired glucose tolerance and reduced number of insulin receptors |
| Amenorrhea/ Oligomenorrhea | Consistent clinical features of PCOS; may result from high testosterone plasma concentrations |
| Infertility | Occurs in 75% of patients; secondary to ovulatory failure |
| Hirsutism | Inconsistent manifestation; may be due to increased peripheral androgen activity at pilosebaceous units of skin |
| Acanthosis nigricans | Associated with obesity; due to insulin resistance |
| Acne/alopecia | Associated with hyperandrogenism in PCOS |
Amenorrhea and oligomenorrhea
are consistent clinical features of PCOS and
have been hypothesized to occur secondary to
high testosterone plasma concentrations.43
However, not all women exhibit dysfunctional
uterine bleeding; some do ovulate and have
regular menstrual cycles.4
Infertility has been documented as a clinical
feature in 75% of women with PCOS.4
The infertility is secondary to ovulatory
failure, which is indicated by low serum
concentrations of pregnanediol.35
Pregnanediol is formed during the metabolism
of progesterone and is found in the urine
during pregnancy and in certain phases of the
menstrual cycle.41
Nevertheless, the normal effect of estrogens
on gonadotropins is maintained in women with
PCOS, supporting the thought that the
hypothalamic and pituitary responses to
estrogen are not a cause of anovulation in
PCOS.35
Furthermore, progesterone serum concentrations
(normally elevated in the luteal phase of the
menstrual cycle) and the presence of
amenorrhea and/or oligomenorrhea determine
chronic anovulation.33
Also, irregular patterns of FSH secretion may
be due to abnormally elevated estrogen serum
concentrations and a causative factor in
anovulation.34
The appearance of hirsutism in women with PCOS
may reflect an increased amount of peripheral
androgen activity at the pilosebaceous units
of the skin.36
The degree of hirsutism can be rated according
to the Ferriman-Gallwey system, from which a
score of at least 8 indicates hirsutism.44
Hirsutism is not a consistent manifestation of
PCOS; women with hyperandrogenemia do not
always exhibit clinical hyperandrogenism.33
Acanthosis nigricans (papillomatous
hyperpigmentation of the epidermal skin layer,
especially in the axilla regions and the neck)
appears to be associated with obesity in women
with and without PCOS30,37;
insulin resistance is the underlying cause of
this condition.37
This skin lesion can also occur in nonobese
women.9,30
Women with PCOS and clincially evident
ancanthosis nigricans have higher insulin
serum concentrations than do women without
this dermatologic problem.9
Nevertheless, hyperinsulinemia can exist in
women with PCOS in the absence of acanthosis
nigricans and obesity.10
A study performed by Dunaif and her colleagues
showed that acanthosis nigricans occurred in
the majority (14 out of 18) of obese women
with PCOS.9
Other clinical features associated with
hyperandrogenism in PCOS include acne and
alopecia.3
Morbidities Associated with PCOS
PCOS can result in significant morbidities,
such as ovarian cancer, coronary artery
disease, and impaired fertility (TABLE 4).
Obesity in women with PCOS is usually
exhibited as an increased waist to hip ratio,
which predisposes women to metabolic health
risks.3 In
fact, it has been estimated that 20% of obese
women with PCOS develop diabetes mellitus or
impaired glucose tolerance (IGT) by their
third decade of life.9,37
High insulin serum concentrations increase the
risk of developing diabetes mellitus (DM) and
therefore, coronary artery disease.
|
Table 4 |
|
• Diabetes
mellitus • Coronary artery disease Myocardial infarction Unstable angina Congestive heart failure • Cardiovascular and metabolic disorders Hypertension Type 2 diabetes Impaired glucose tolerance • Increased risk for endometrial cancer • Possible association for ovarian cancer Source: Based on references 6, 9, 37, 45, 46 |
Cardiovascular and metabolic disorders,
specifically hypertension, type 2 diabetes
mellitus, and impaired glucose tolerance, can
occur in women with PCOS at a greater rate
than in normal women, some investigators
believe. This increased risk for complications
is due to the insulin resistance and obesity
that are common characteristics in women with
PCOS. PCOS also has been shown to reduce HDL
and increase triglyceride plasma
concentrations, which further compounds the
cardiovascular risk in these women.6
Young women with PCOS need to be closely
monitored for the development of impaired
glucose tolerance and diabetes mellitis. DM is
a major contributor to the risk of coronary
artery disease, which can manifest as
myocardial infarction, unstable angina,
congestive heart failure, and other
life-threatening conditions.45
Hypertension is another complication of PCOS.
Young women with PCOS generally have blood
pressure readings within the normal range;
with increasing age, however, systolic blood
pressure also increases. The elevation in
systolic blood pressure is higher in women
with PCOS than in age-matched controls.45
Women with PCOS are at increased risk for
endometrial cancer secondary to prolonged,
unopposed estrogen stimulation of the
endo-metrium.6
An association between ovarian cancer and PCOS
also has been suggested by Schildkraut and
colleagues.46
Treatment
Generally, therapy attempts to relieve the
particular complaint of the patient, which can
vary from infertility and/or menstrual
dysfunction to physical appearance; on the
other hand, if medical treatment to enhance
insulin sensitivity is begun early, then the
long-term outcome would be much improved.3
Weight Reduction: The first
line of therapy in obese women with PCOS is
weight reduction to reduce hyperinsulinemia
and its effects on hyperandrogenemia. Weight
reduction in obese women increases SHBG and
decreases insulin resistance, leading to
lowered androgen plasma concentrations.
Successful obesity management may restore
ovulation in women with PCOS. Other benefits
are associated with decreasing insulin and
androgen serum concentrations, including
improvement of hirsutism and acanthosis
nigricans.3
Antiandrogens: Spironolactone
blocks the binding of DHT (the active moiety
of testosterone) to androgen receptors,
causing regression of hair growth. This agent
also decreases p450c17alpha activity, which
reduces androgen production. Given at a daily
divided dosage of 100¡V200 mg for 6¡V12 months,
spironolactone has been shown to reduce hair
growth.47-48
Adverse effects of spironolactone include
hyperkalemia, dehydration, and hyponatremia.49
Finasteride (4-azasteroid), a 5alpha-reductase
inhibitor, reduces the conversion of
testosterone to DHT, thereby decreasing the
binding of DHT to the androgen receptor.50,51
It has been shown to be as effective as
spironolactone in decreasing the hair shaft
diameter.50
PCOS patients treated with finasteride for 12
months had a statistically significant
reduction in their Ferriman-Gallwey score,
with substantial improvement noted in their
hirsutism scores by 3 months of treatment and
maximal response at 6 months. The most common
adverse effect was mild and transient nausea.51
The antiandrogen flutamide competitively
inhibits binding of testosterone to androgen
receptors.49
It is primarily used in the treatment of
prostatic carcinoma. This agent should only be
used in women with PCOS when other therapies
for hirsutism did not prove to be effective.
Ovulation Inducers: A number
of agents may be considered for inducing
ovulation in women with PCOS.
• Oral contraceptives:
Various reports demonstrate the beneficial
effects of using an oral contraceptive (OC) in
women with PCOS. OCs have been postulated to
decrease LH, increase hepatic SHBG production,
and inhibit receptor binding of
5alpha-reductase and androgens.52-53
Givens et al.54
used an OC containing 2 mg norethindrone and
0.1 mg mestranol, given on a cyclic basis to a
17-year-old woman with PCOS and a stromal
luteoma. The OC decreased her plasma
concentrations of androstenedione and
testosterone, and improved her acanthosis
nigricans almost to the point of
disappearance.
It is important to use OCs with progestational
components with minimal androgenic potency,
such as those with desogestrel or norgestimate.
Agents with progestins of high androgenic
potency¡Xi.e., levonorgestrel and norethindrone¡Xshould
be avoided in women with PCOS. These agents
have negative effects on the lipid profile
such that HDL decreases and LDL increases,
whereas desogestrel and norgestimate have
positive effects on lipids.49
Oral contraceptives also help treat acne and
hirsutism and prevent ovarian and endometrial
cancer.3
• Cyproterone acetate:
A common agent for treating PCOS outside the
U.S., cyproterone acetate (CPA) has
progestinic, anti-androgenic, and mild
glucocorticoid activity. It suppresses ovarian
steroidogenesis, decreases plasma testosterone
concentrations, and induces hepatic
metabolism. Amenorrhea may result if CPA is
used for longer than the first 10 days of the
OC pill cycle. (It is manufactured in a
combination tablet with ethinyl estradiol and
as a single agent for the treatment of
prostate cancer.) Weight gain and edema can
result and may be due to its glucocorticoid
activity.55
CPA has been used successfully in combination
with ethinyl estradiol and leuprolide acetate
to reduce hirsutism in women who did not
respond to OC treatment alone.56
The use of an OC tablet containing ethinyl
estradiol-CPA with or without a GnRH agonist
caused significant reductions in
Ferriman-Gallwey scores, estradiol,
testosterone, androstenedione, and 17-OH
progesterone serum concentrations. The effect
on gonadotropins was more pronounced in the
group that received the GnRH agonist. There
was a greater decrease in hirsutism in the
obese and hirsute groups in this trial. GnRH
agonist therapy should be reserved for use in
obese women with severe hirsutism because of
its expense and greater effectiveness in this
patient population.57
• Clomiphene citrate:
A racemic compound with estrogen agonist and
antagonist activity, clomiphene citrate¡¦s
activity is determined by the dose used and by
the recipient¡¦s endogenous estrogenic status.58
It induces ovulation by increasing the pulse
frequency of GnRH (i.e., the occurrence of
increased GnRH release from the hypothalamus).
It is effective in women with PCOS because
|
Clomiphene citrate can be a first-line treatment for stimulating ovulation in women with PCOS. |
it decreases LH and increases
SHBG serum concentrations.59
Therapy is initiated at an oral
dose of 50 mg per day for 5 days in the early
follicular phase. The dose can be increased to
100 mg and then to 150 mg if ovulation fails
to occur with the lower dose. The lowest
possible dose should be used. Generally, if
the 150 mg dosage is not effective, then
another therapy is instituted.58
Adverse effects include hot flushes, nausea,
vomiting, and ovarian hyperstimulation.
Interestingly, one isomer of CC is
structurally related to diethylstilbestrol,
and CC should not be used in early pregnancy.
However, CC is still considered an effective
first-line treatment for stimulating ovulation
in women with PCOS.58
• Gonadotropins:
Human chorionic gonadotropin (hCG) and human
menopausal gonadotropin (hMG) are used to
stimulate ovulation in women who do not
respond to clomiphene citrate. When given in
combination with CC, hCG can be administered
at a dose of 5000 IU to induce ovulation.
Clomiphene¡¦s use with hMG is usually to reduce
the dose of gonadotropin given in order to
decrease the risk of hyperstimulation and
high-order multiple pregnancy.58
In one study,60
a total dose of 300 IU of hMG was initiated
for 3 days, then the dosage adjusted based on
the rise of serum estradiol concentrations.
When a leading follicle obtained the desired
size, then 10,000 units of hCG was
administered intramuscularly to achieve
ovulation.
• GnRH agonists:
Taskin and colleagues evaluated the effect of
subcutaneously administered goserelin acetate
and OC treatment, versus ovarian
cauterization, on the biochemical profile of
women with clomiphene-resistant PCOS. Both
modalities decreased LH, FSH, androstenedione,
and testosterone, and increased SHBG serum
concentrations. However, the oral combination
caused a greater reduction in LH and elevation
in SHBG,57
was less invasive and expensive, and is not as
likely to cause infertility as a consequence
of surgically acquired periovarian adhesions.
The available GnRH agonists in the U.S. are
goserelin acetate, leuprolide acetate, and
nafarelin acetate.49,61
Adverse effects include decreased bone mineral
density with prolonged use, hot flashes,
decreased libido and ovarian hyperstimulation
syndrome (OHSS). Abdominal ascites and ovarian
enlargement are the main characteristics of
OHSS. The acute fluid overload can cause
respiratory distress and even pulmonary edema.
Renal failure, stroke, and death can result
from this dangerous adverse effect,
particularly in women with PCOS.62
These agents are contraindicated in pregnancy
because of its teratogenicity in animals.49,61
• Glucocorticoids:
Glucocorticoids are an option for adjunctive
therapy to induce ovulation in women with PCOS
who do not respond to clomiphene alone.
Glucocorticoids reduce adrenal androgen
secretion, which increases the likelihood of
ovulation and pregnancy.63
Singh and colleagues evaluated clomiphene and
dexamethasone in women with PCOS. Therapy was
initiated at a CC dose of 50 mg plus
dexamethasone 0.5 mg on day 5 of the menstrual
cycle. The dose of
|
Therapies for PCOS aim to reduce androgen secretion, improve fertility, and manage insulin resistance. |
clomiphene was titrated to 150
mg per day in women who did not respond to
lower doses. The concurrent use of clomiphene
and dexamethasone in women with clomiphene
resistance resulted in ovulation in 88.8% of
the women with PCOS.64
Insulin Sensitizers:
Insulin sensitizers¡Xspecifically, metformin
and troglitazone65-69¡Xhave
been used in women with PCOS with positive
results. However, troglitazone has been
removed from the market because of hepatotoxic
effects that caused liver failure and death.
Metformin is a biguanide antidiabetic agent
that is not FDA-approved for use in the
treatment of PCOS. Nevertheless, it is
beneficial in managing the insulin resistance
that is a common characteristic in these
patients. Metformin does not affect insulin
secretion, but it does decrease hepatic
glucose production and improve peripheral
glucose utilization.49
It has been demonstrated to reduce unbound
testosterone and androstenedione,65,67
total testosterone,66
and fasting insulin serum concentrations,67
and increase SHBG65-66
and FSH67
plasma levels. Its positive effects on insulin
resistance and reduced free testosterone have
helped to increase successful pregnancy rates
in women with PCOS.70
A dose of metformin at 500 mg given three
times daily has been shown to be effective
when used in managing PCOS.70
Metformin¡¦s most common side effects include
nausea, diarrhea, and abdominal discomfort.
Patients with renal insufficiency should not
take this medication because of the increased
risk of lactic acidosis. Metformin should not
be used in women who are pregnant.49
Surgery: In general,
operative techniques to manage this syndrome
are used following treatment failure with
clomiphene, gonadotropin, and LHRH. Ovarian
wedge resection was the first surgical
maneuver described. It is successful in
restoring ovulation because of the destruction
of excess ovarian stroma, which decreases the
amount of tissue available for androgen
conversion to estrogen. However, this
procedure is associated with a high risk of
periadnexal adhesion formation.71
Unilateral oophorectomy should be restricted
for use in women with concomitant ovarian
pathology. Fortunately, this procedure does
not cause periadnexal adhesion formation.71
Ovarian drilling by laparoscopy is also known
as laparoscopic ovarian diathermy,
laparoscopic ovarian electrocautery, and
laparoscopic electrocoagulation. An electric
current is used in this procedure, causing
release of follicular fluid that contains
large amounts of androgens. Risks of this
surgery include thermal injury to surrounding
tissues, periadnexal adhesion formation, and
premature ovarian failure.71
The
Pharmacist¡¦s Role
Pharmacists are ideally situated to aid the
healthcare team in instituting cardiovascular
preventive measures in women with polycystic
ovary syndrome. Women who are obese can be
counseled by pharmacists in techniques for
weight loss and assessed for possible adverse
effects from treatment. Pharmacists in an
outpatient setting are able to remind patients
on a regular basis about the importance of
exercise and meal planning to reduce weight
and improve their lipid profile.
Counseling patients about preventing
hypertension should include instructions on
implementing a low-salt diet, an exercise
regimen, maintaining a nonobese weight, and
cessation of smoking, when applicable. These
are necessary components of a pharmacist¡¦s
service to patients with polycystic ovary
syndrome.
Conclusion
According to one source, ¡§PCOS is a fugitive
syndrome with limits less well defined than
those of the Sahara or Sudan.¡¨72
Recent literature supports the role of
hyperinsulinemia as one of the major
pathogenic factors causing the hyperandrogenic
manifestations associated with polycystic
ovary syndrome. Healthcare practitioners must
be aware of the morbidities associated with
insulin resistance, and hence PCOS, in order
to help prevent their occurrence. Therapies
for women with polycystic ovary syndrome
target reduced androgen secretion, regulation
of gonadotropin release, improvement of
fertility, and management of insulin
resistance. The newest agent, metformin, has
been demonstrated to improve all of the
aforementioned parameters as a solo agent for
managing PCOS.
Oral contraceptives are useful in hirsute
women who do not want to conceive a child.
Adjunctive management of hirsutism includes
hair removal with depilatories, shaving,
electrolysis, and the use of medical therapy
as discussed above. If a woman is not hirsute
and does not want to become pregnant, use of
medroxyprogesterone acetate (MPA) for 10 days
each month is a necessity to cause withdrawal
vaginal bleeding. The benefit of this action
is to prevent endometrial hyperplasia, which
can lead to endometrial cancer.41
If pregnancy is desired, then clomiphene
citrate is the first line-therapy. If
clomiphene does not work, then use of a GnRH
agonist is a reasonable option after a trial
of gonadotropin. Other options for inducing
ovulation include glucocorticoids and
metformin. Cauterization or laser may be
utilized for ovarian drilling to induce
ovulation in women who do not respond to
hormonal treatment. However, ovarian adhesions
from surgical procedures is a common
occurrence.