ˇ@
Gestational trophoblastic tumors (GTTs) encompass a spectrum of neoplastic disorders that arise from placental trophoblastic tissue after abnormal fertilization. GTTs are classified histologically into four distinct groups: hydatidiform mole (complete and partial), chorioadenoma destruens (invasive mole), choriocarcinoma, and placental site tumor. Most commonly, GTT results in a hydatidiform "molar" pregnancy characterized by the lack of a fetus, trophoblastic hyperplasia, edematous chorionic villi, and a loss of normal villous blood vessels.
Most molar pregnancies spontaneously resolve after uterine evacuation with no further sequelae. However, at any time during or after gestation, malignant transformation may occur in approximately 10%-20% of molar pregnancies. Nearly two thirds of these cases have an invasive mole confined to the uterus (chorioadenoma destruens). Choriocarcinoma characterized by distant metastatic spread develops in one third of patients. Placental site tumors are uncommon neoplasms derived from intermediate trophoblast cells of the placenta, which are identified by cellular secretion of placental lactogen and small amounts of b-subunit human chorionic gonadotropin (b-hCG).
In the United States, GTTs account for less than 1% of gynecologic malignancies. However, knowledge of the natural history and management of GTTs is important because of this tumor’s potential for cure with appropriate therapy. Forty years ago, women with choriocarcinoma had a 95% mortality rate. Today, with the advent of effective chemotherapy and the development of a reliable tumor marker (b-hCG), the cure rate for choriocarcinoma is 90%-95%.
A hydatidiform mole develops in approximately 1 in 1,000-2,000 pregnancies in the United States. Molar pregnancies are reported in approximately 3,000 patients per year, and malignant transformation occurs in 6%-19% of these cases. Complete molar pregnancies occur in 1 in 40 molar pregnancies, 1 in 15,000 abortions, and 1 in 150,000 normal pregnancies. Overall, approximately 80% of cases of GTTs are hydatidiform moles, 15% are chorioadenoma destruens, and 5% are choriocarcinomas. Choriocarcinoma is associated with an antecedent mole in 50% of cases, a history of abortion in 25%, term delivery in 20%, and ectopic pregnancy in 5%.
PATHOLOGY
Hydatidiform moles, invasive moles, and choriocarcinomas have distinct morphologic features. Moles are described as partial or complete (classic) based on their morphologic, karyotypic, and clinical features. Complete moles are distinguished by the complete absence of normal villi and by chromosomal material that is virtually always of paternal origin. Partial moles are characterized grossly by an admixture of normal and hydropic villi, a triploid karyotype, and the presence of both maternal and paternal chromosomal material.
Choriocarcinomas have a unique histology that is distinct from that of the moles. The tumor is grossly red and granular and exhibits extensive necrosis and hemorrhage. Microscopically, the neoplasm is composed of a disordered array of syncytiotrophoblastic and cytotrophoblastic elements, frequent mitoses, and multinucleated giant cells. Vascular invasion occurs early, with resultant metastases to the lungs, vagina, brain, kidneys, liver, and gastrointestinal tract.
CLINICAL PRESENTATION
Complete mole
The classic signs of a molar pregnancy include the absence of fetal heart sounds, physical evidence of a uterus that is larger than expected for gestational age, and vaginal bleeding. Although an intact fetus may coexist with a partial mole, this occurs in fewer than 1 in 100,000 pregnancies.
The most common presenting symptom of molar pregnancy is vaginal bleeding, reported in up to 97% of patients. Intrauterine clots may undergo oxidation and liquefaction, producing pathognomonic prune juice-like fluid. Rarely, spontaneous expulsion of grape-like villi will provide the diagnosis of hydatidiform mole. Prolonged or recurrent bleeding may result in iron-deficiency anemia. Symptoms of anemia occur in approximately 50% of patients at the time of diagnosis.
Early toxemia (hypertension, proteinuria, and edema) presenting during the first or second trimester is common (20%-30%) in molar pregnancy. Very rarely, eclamptic convulsions may occur in this setting.
Hyperemesis gravidarum—protracted nausea and vomiting during pregnancy—is observed in approximately 10% of women with GTT. The mechanism is not well understood.
Hyperthyroidism is seen clinically in approximately 7% of molar pregnancies. An elevation of triiodothyronine (T3) and thyroxine (T4) levels is observed more commonly than are the manifestations of tachycardia, sweating, weight loss, and tremor. These hormonal elevations are presumed to be secondary to the structural similarity of hCG to thyroid-stimulating hormone (TSH).
Partial mole
Patients with partial mole have different clinical features than those with complete mole. Fewer than 10% of patients with partial mole have uterine enlargement. Patients with partial mole do not have prominent theca-luteal cysts, hyperthyroidism, or respiratory insufficiency. They experience toxemia only rarely. The diagnosis of partial mole is usually made after histologic review of curettage specimens.
Metastatic trophoblastic disease
Metastatic GTT is reported in 6%-19% of patients after molar evacuation. Metastases sometimes have an identical histology to that of molar disease, but the vast majority are choriocarcinomas. Metastatic spread is hematogenous. Because of its extensive vascular network, metastatic GTT often produces local, spontaneous bleeding. Berkowitz et al at the New England Trophoblastic Disease Center (NETDC) reported that the common metastatic sites of GTT are the lungs (80%); vagina (30%); pelvis (20%); liver (10%); brain (10%); and bowel, kidneys, and spleen (5% each).
Pulmonary metastases are quite common (80% of patients with metastatic disease) and occur when trophoblastic tissue enters the circulation via uterine venous sinuses. The radiologic features may be protean or subtle and include alveolar, nodular, and miliary patterns. Pleural effusions may also be present. Pulmonary metastases can be extensive and can cause respiratory failure and death.
Right upper-quadrant pain has been observed when hepatic metastases stretch Glisson’s capsule. Gastrointestinal lesions may result in severe hemorrhage or in perforation with peritonitis, both of which require emergency intervention. Vaginal examination may reveal bluish metastatic deposits; these and other metastatic sites should not undergo biopsy because severe uncontrolled bleeding may occur.
Central nervous system (CNS) involvement from metastatic GTT suggests widespread disease and has a poor prognosis. CNS metastases are clinically evident in 7%-28% of patients with metastatic choriocarcinoma. Bakri and colleagues reported a 17% incidence of patients with GTT metastatic to the brain. All patients had concurrent pulmonary metastases. Cerebral metastases tend to respond favorably to both radiotherapy and chemotherapy.
DIAGNOSTIC STUDIES
Although the clinical presentation may suggest a diagnosis of GTT, certain laboratory studies, particularly a determination of the patient’s b-hCG level, and radiographic studies are needed to confirm this diagnosis.
Laboratory studies
Thyroid function studies should be performed in all patients with a clinical history or physical examination suggestive of hyperthyroidism. Abnormal thyroid function, manifested as an elevated T4 level, is common in GTT. Metastatic deposits in the kidneys or gastrointestinal tract may reveal themselves by hematuria or hematochezia.
Tumor markers A well-characterized glycoprotein hormone secreted by the syncytiotrophoblast, hCG is essential to maintaining normal function of the corpus luteum during pregnancy. This hormone has an a-subunit identical to the a-subunit of the pituitary hormones and a b-subunit (b-hCG) that confers the hormone’s unique biologic activity. The presence of hCG appears approximately 8 days after ovulation, and its concentration doubles every 2-4 days, until it peaks at 10-12 weeks of gestation; thereafter, b-hCG levels decline steadily. Because all trophoblastic tumors secrete b-hCG, this hormone serves as an excellent marker for tumor activity in the nonpregnant patient.
Serial b-hCG levels should be monitored during therapy to ensure adequate treatment. The level of b-hCG is roughly proportional to the tumor burden and inversely proportional to therapeutic outcome. The approximately 10%-20% of patients with hydatidiform mole who are not cured by local therapy or do not achieve a spontaneous remission can be identified by a rising or plateauing b-hCG titer on serial determinations after the evacuation of a mole. These patients are considered to have persistent trophoblastic disease and require additional therapy.
Note: In an occasional patient the current b-hCG assays may result in a false elevation. This may be secondary to a nonspecific cross-reaction between antibodies in patients serum and in the immunoassay kit, similar to that initially observed in CA-125 assays after murine antibody administration. Most commonly these are human antimouse antibodies (HAMA). As always in medicine, if the laboratory result does not fit the clinical picture it needs to be verified using alternative methods.
RADIOLOGIC STUDIES
A chest x-ray should always be performed because 70%-80% of patients with metastatic GTT have lung involvement. Although this x-ray usually demonstrates nodular metastases, the patterns of metastatic disease can range from atelectatic areas to subtle pleural abnormalities. A CT scan often is helpful in evaluating these nonspecific findings.
Since it has been demonstrated that 97%-100% of patients with CNS disease from choriocarcinoma have concomitant pulmonary metastases, a CNS work-up in asymptomatic patients with normal chest x-rays is not routinely warranted. If the chest x-ray is abnormal, or if b-hCG levels plateau or rise during treatment, a more thorough evaluation for metastatic disease is indicated. Magnetic resonance imaging (MRI) of the brain, brain stem and cerebellum, CT scans of the abdomen and pelvis should be performed to evaluate other likely sites of metastatic spread. The presence of intrauterine or ovarian disease also may be detected by MRI of the pelvis.
Ultrasonography is a reliable, safe, economical, and relatively simple method for confirming the diagnosis of intrauterine GTT. It is also useful in identifying embryonic remnants.
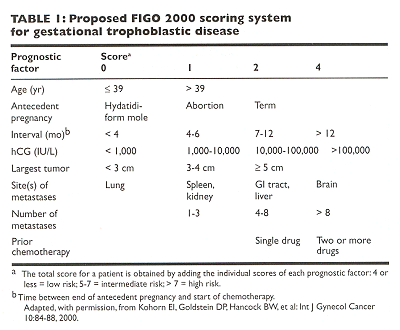
The proposed FIGO 2000 anatomic staging system is a
straightforward system based on anatomic criteria. In GTT, stage I disease is
confined to the uterus; stage II disease is outside the uterus but limited to
the genital structures. Stage III disease extends to the lungs with or without
known genital tract involvement, whereas stage IV disease includes all other
metastatic sites. The FIGO 2000 scoring system (Table ) is based on a method (adapted
from WHO) to identify patients at high risk for treatment failure. With the FIGO
2000 scoring system, patients are classified as being in a low-, middle-, or
high-risk category. A total score of up to 4 is considered low risk; 5-7, middle
risk; and 8 or greater, high risk. (Some centers recommend a low-risk score of 6
or less, a high-risk score of 7 or greater, and no middle-risk score.)
ˇ@
TREATMENT
Although the treatment strategy for GTT must be individualized for each patient, Figure summarizes the general diagnostic and therapeutic approaches used at The University of Texas M. D. Anderson Cancer Center. The stratification of risk groups enables physicians to direct an appropriate treatment strategy. Low-risk disease responds readily to single-agent chemotherapy and is virtually 100% curable. High-risk disease is not likely to be cured with single-agent therapy and therefore requires multidrug regimens.

Molar pregnancy
For patients with complete or partial hydatidiform mole, evacuation of the mole by suction and sharp curettage should be performed. Oxytocics also are given to produce uterine involution and to control bleeding. However, these agents should be used judiciously as they may cause hyponatremia and fluid overload. A baseline chest x-ray and b-hCG measurement should be obtained prior to surgery.
After molar evacuation, 80% of patients will need no further intervention. However, these patients?weekly serum b-hCG levels must be diligently monitored until they return to normal. Although normal b-hCG levels typically return within 8 weeks of surgery, in a minority of patients it takes 14-16 weeks for levels to return to normal. Sometimes transient plateaus are observed before the b-hCG level returns to baseline. An increased or prolonged plateau of b-hCG titers implies persistent trophoblastic disease or metastatic spread and requires additional therapy.
Chemotherapy is indicated when there is a plateau or increase in b-hCG levels on consecutive measurements, failure to reach normal titers by 16 weeks, or metastatic disease. Such patients are usually at low risk and will respond to single-agent chemotherapy. Methotrexate is the most commonly initiated single agent (Table 2). Therapy is continued for one to two courses after a normal b-hCG level is achieved.
Follow-up As mentioned previously, all patients with molar disease should obtain a baseline chest x-ray. Serial b-hCG levels should be obtained every 1-2 weeks until the level is normal for three consecutive assays. Complete remission is defined by three consecutive normal b-hCG levels. Once this has occurred, b-hCG levels should be checked monthly for 12 months, every 4 months for the following year, and then yearly for 2 years.
Although the use of oral contraceptives during the surveillance period remains controversial, strict contraception is required, because pregnancy would obviate the usefulness of b-hCG as a tumor marker. In general, once a 12-month surveillance establishes a disease-free status, conception is acceptable. These women are always at high risk for future molar disease and will require close observation during future pregnancies. A pelvic ultrasound examination should be performed during the first trimester of all subsequent pregnancies to confirm that gestation is normal.
LOW-RISK METASTATIC DISEASE
In more than 30 years of experience, single-agent chemotherapy with metho-trexate has produced a high cure rate in patients with low-risk GTT. Likewise, methotrexate plus folinic acid (leucovorin) induces remission in 90% of patients with low-risk metastatic disease with low toxicity. The use of dactinomycin in methotrexate-resistant patients increased the cure rate to more than 95%.
Suggested therapeutic regimens for low-risk GTT are outlined in Table . The regimen of intramuscular methotrexate plus leucovorin is preferred because it obviates intravenous access problems, allows the therapeutic administration at home or work, minimizes the interruption of the patient’s life, and is the least toxic of the regimens listed. These patients are usually treated for two to three courses after attaining a normal b-hCG level.

If single-agent therapy with methotrexate or dactinomycin fails to achieve remission, multidrug chemotherapy must be attempted. This is necessary in nearly 40% of patients with low-risk metastatic GTT. Despite resistance to first-line chemotherapy, a cure rate of almost 100% is achieved with further combination chemotherapy.
HIGH-RISK METASTATIC DISEASE
The discovery that etoposide is an effective agent against trophoblastic disease led to the development of the EMA-CO regimen by Bagshawe, who reported a survival rate of 83% in patients with high-risk choriocarcinoma. This regimen has been confirmed to be highly effective at several centers, including the Brewer Trophoblastic Disease Center, where a 100% cure rate has been achieved over the past 5 years.
EMA-CO (Table ) is the preferred regimen for high-risk GTT. We also utilize this regimen for patients with middle-risk GTT, as defined by the FIGO 2000 criteria. EMA-CO is generally well tolerated, with no life-threatening toxic effects. Alopecia occurs universally, and anemia, neutropenia, and stomatitis are mild. Reproductive function is preserved in approximately 75% of patients.
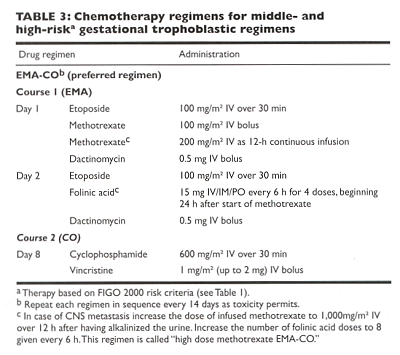
ˇ@
Within hours of receiving chemotherapy, patients with a significant tumor burden are at risk of hemorrhage into tumors and surrounding tissues. Thus, any acute organ toxicity that begins shortly after the induction of chemotherapy should be considered as possibly related to this phenomenon. Some researchers have advocated a reduction in dosage at the beginning of therapy in patients with large-volume disease to minimize these sequelae.
SALVAGE THERAPY
Unfortunately, about 25% of women with high-risk metastatic disease become refractory to EMA-CO and fail to achieve a complete remission. Currently, there is no standard salvage chemotherapy regimen for patients not responding to EMA-CO. Salvage regimens which combine cisplatin (Platinol), etoposide, vinca alkaloids, and bleomycin (Blenoxane) have been administered, however.
Because of significant nephrotoxicity, cisplatin-containing regimens are withheld as primary therapy for GTT. Early studies show cisplatin-based regimens to be an effective salvage therapy in GTT. A recent dose-intensive regimen, EMA-CE, utilizes cisplatin (100 mg/m2) and etoposide (200 mg/m2) combined with EMA, with favorable results.
Another alternative is to give cisplatin in the EMA-POMB regimen (Platinol [cisplatin], Oncovin [vincristine], methotrexate, bleomycin). POMB is administered as vincristine, 1 mg/m2 IV, and methotrexate, 300 mg/m2 IV (day 1); bleomycin, 15 mg IV over 24 h by continuous infusion (CI), and folinic acid, 15 mg bid for four doses (day 2); bleomycin, 15 mg IV over 24 h CI (day 3); and cisplatin, 120 mg/m2 IV (day 4).
A new PEBA regimen (Platinol [cisplatin], etoposide, bleomycin, Adriamycin [doxorubicin]) was recently reported from China and was found to be effective in EMA-CO-resistant disease. A complete remission (CR) was achieved in 96% of the women, and 73% had a sustained CR that lasted at least 1 year. In a small study, ifosfamide (Ifex) alone and combined in the VIP regimen (VePesid [etoposide], ifosfamide and Platinol [cisplatin]) showed promise as being an effective salvage drug in GTT.
Another consideration in the treatment of refractory GTT is the use of high-dose chemotherapy with autologous bone marrow transplantation. In this setting, Lotz et al treated five women with refractory GTT with high-dose ifosfamide, carboplatin (Paraplatin), and etoposide (ICE). Only one of the five women attained a durable CR (68+ months). The risk and benefits of high-dose chemotherapy in the treatment of GTT is still under investigation.
PULMONARY METASTASES
At the time of diagnosis, pulmonary metastases can be extensive and may cause respiratory failure and death.
In patients with extensive pulmonary metastases, reduced doses of initial chemotherapy (eg, 50%) have been suggested to diminish the risk of respiratory failure. However, reduction of the initial chemotherapy dose did not uniformly protect against pulmonary failure and death. Because of the increased risk of pulmonary decompensation, women with extensive pulmonary metastases should be observed in an intensive care setting during induction chemotherapy.
CNS CHORIOCARCINOMA
Brain metastases pose a significant threat to the survival of patients with GTT, especially if the metastases appear while the patient is receiving chemotherapy. Although cerebral disease is observed clinically in only 7%-28% of patients with choriocarcinoma, postmortem examinations demonstrate CNS involvement in as many as 40% of cases. This subset represents a significant fraction of patients who die of the disease.
In 1983, Athanassiou et al reported results of a 23-year experience with choriocarcinoma involving the CNS at Charing Cross Hospital. Overall, 8.8% of 782 patients with choriocarcinoma who received chemotherapy had CNS metastases. Of these patients, 48% presented with CNS disease prior to treatment. Although 49% of patients who presented with CNS metastases enjoyed long-term survival, only 6% of the patients who developed CNS disease while on therapy survived. Radiotherapy did not appear to benefit patients whose disease was resistant to chemotherapy.
Page RD, Kudelka AP, Freedman RS, et al: Gestational trophoblastic tumors, in Pazdur R (ed): Medical Oncology, A Comprehensive Review, PRR, Huntington, New York, pp 377?91, 1995 (also on Cancer Information Network website: http://www.cancernetwork.com/).
Kohorn EI, Goldstein DP, Hancock BW, et al: Combining the staging system of the International Federation of Gynecology and Obstetrics with the scoring system of the World Health Organization for trophoblastic neoplasia. Report of the Working Committee of the International Society for the Study of Trophoblastic Disease and the International Gynecologic Cancer Society. Int J Gynecol Cancer 10:84?8, 2000.
Li-Pai C, Shu-Mo C, Jian-Xuan F, et al: PEBA regimen (cisplatin, etoposide, bleomycin, and Adriamycin) in the treatment of drug-resistant choriocarcinoma. Gynecol Oncol 56:231?34, 1995.
Lotz JP, Andre T, Donsimoni R, et al: High-dose chemotherapy with ifosfamide, carboplatin, and etoposide combined with autologous bone marrow transplantation for the treatment of poor-prognosis germ cell tumors and metastatic trophoblastic disease in adults. Cancer 75:874?85, 1995.