|
I. Embryology |
|
|
|
THE TERM Embryology, in its widest sense, is applied to
the various changes which take place during the growth of an animal from the
egg to the adult condition: it is, however, usually restricted to the
phenomena which occur before birth. Embryology may be studied from two
aspects: ( |
|
|
In vertebrate animals the development of a new being can
only take place when a female germ cell or ovum has been fertilized by
a male germ cell or spermatozoön. The ovum is a nucleated cell, and
all the complicated changes by which the various tissues and organs of the
body are formed from it, after it has been fertilized, are the result of two
general processes, viz., segmentation and differentiation of
cells. Thus, the fertilized ovum undergoes repeated segmentation into a
number of cells which at first closely resemble one another, but are, sooner
or later, differentiated into two groups: ( |
|
|
Having regard to the main purpose of this work, it is
impossible, in the space available in this section, to describe fully, or
illustrate adequately, all the phenomena which occur in the different stages
of the development of the human body. Only the principal facts are given, and
the student is referred for further details to one or other of the text-books 1
on human embryology. |
|
|
|
|
|
|
|
|
|
|
|
All the tissues and organs of the body originate from a
microscopic structure (the fertilized ovum), which consists of a soft
jelly-like material enclosed in a membrane and containing a vesicle or small spherical
body inside which are one or more denser spots. This may be regarded as a
complete cell. All the solid tissues consist largely of cells essentially
similar to it in nature but differing in external form. |
|
|
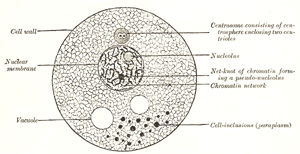
In the higher organisms a cell may be defined as “a
nucleated mass of protoplasm of microscopic size.” Its two essentials,
therefore, are: a soft jelly-like material, similar to that found in the
ovum, and usually styled cytoplasm, and a small spherical body
imbedded in it, and termed a nucleus. Some of the unicellular protozoa
contain no nuclei but granular particles which, like true nuclei, stain with
basic dyes. The other constituents of the ovum, viz., its limiting membrane
and the denser spot contained in the nucleus, called the nucleolus,
are not essential to the type cell, and in fact many cells exist without
them. |
|
|
Cytoplasm (protoplasm) is a material probably
of variable constitution during life, but yielding on its disintegration
bodies chiefly of proteid nature. Lecithin and cholesterin are constantly
found in it, as well as inorganic salts, chief among which are the phosphates
and chlorides of potassium, sodium, and calcium. It is of a semifluid, viscid
consistence, and probably colloidal in nature. The living cytoplasm appears
to consist of a homogeneous and structureless ground-substance in which are
embedded granules of various types. The mitochondria are the most
constant type of granule and vary in form from granules to rods and threads.
Their function is unknown. Some of the granules are proteid in nature and
probably essential constituents; others are fat, glycogen, or pigment
granules, and are regarded as adventitious material taken in from without,
and hence are styled cell-inclusions or paraplasm. When, however, cells
have been “fixed” by reagents a fibrillar or granular appearance can often be
made out under a high power of the microscope. The fibrils are usually
arranged in a network or reticulum, to which the term spongioplasm is
applied, the clear substance in the meshes being termed hyaloplasm.
The size and shape of the meshes of the spongioplasm vary in different cells
and in different parts of the same cell. The relative amounts of spongioplasm
and hyaloplasm also vary in different cells, the latter preponderating in the
young cell and the former increasing at the expense of the hyaloplasm as the
cell grows. Such appearances in fixed cells are no indication whatsoever of
the existence of similar structures in the living, although there must have
been something in the living cell to give rise to the fixed structures. The
peripheral layer of a cell is in all cases modified, either by the formation
of a definite cell membrane as in the ovum, or more frequently in the
case of animal cells, by a transformation, probably chemical in nature, which
is only recognizable by the fact that the surface of the cell behaves as a
semipermeable membrane. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Nucleus.—The nucleus is a minute body,
imbedded in the protoplasm, and usually of a spherical or oval form, its size
having little relation to that of the cell. It is surrounded by a
well-defined wall, the nuclear membrane; this encloses the nuclear
substance (nuclear matrix), which is composed of a homogeneous
material in which is usually embedded one or two nucleoli. In fixed cells the
nucleus seems to consist of a clear substance or karyoplasm and a
network or karyomitome. The former is probably of the same nature as
the hyaloplasm of the cell, but the latter, which forms also the wall of the
nucleus, differs from the spongioplasm of the cell substance. It consists of
fibers or filaments arranged in a reticular manner. These filaments are
composed of a homogeneous material known as linin, which stains with
acid dyes and contains embedded in its substance particles which have a
strong affinity for basic dyes. These basophil granules have been named chromatin
or basichromatin and owe their staining properties to the presence of
nucleic acid. Within the nuclear matrix are one or more highly refracting
bodies, termed nucleoli, connected with the nuclear membrane by the
nuclear filaments. They are regarded as being of two kinds. Some are mere
local condensations (“net-knots”) of the chromatin; these are irregular in
shape and are termed pseudo-nucleoli; others are distinct bodies
differing from the pseudo-nucleoli both in nature and chemical composition;
they may be termed true nucleoli, and are usually found in resting
cells. The true nucleoli are oxyphil, i.e., they stain with acid dyes. |
|
|
Most living cells contain, in addition to their protoplasm
and nucleus, a small particle which usually lies near the nucleus and is
termed the centrosome. In the middle of the centrosome is a minute
body called the centriole, and surrounding this is a clear spherical
mass known as the centrosphere. The protoplasm surrounding the
centrosphere is frequently arranged in radiating fibrillar rows of granules,
forming what is termed the attraction sphere. |
|
|
|
|
|
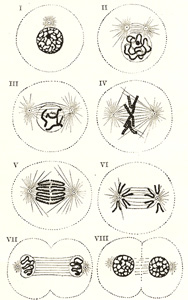
Reproduction of Cells.—Reproduction
of cells is effected either by direct or by indirect division.
In reproduction by direct division the nucleus becomes constricted in
its center, assuming an hour-glass shape, and then divides into two. This is
followed by a cleavage or division of the whole protoplasmic mass of the
cell; and thus two daughter cells are formed, each containing a nucleus.
These daughter cells are at first smaller than the original mother cell; but
they grow, and the process may be repeated in them, so that multiplication
may take place rapidly. Indirect division or karyokinesis (karyomitosis)
has been observed in all the tissues—generative cells, epithelial tissue,
connective tissue, muscular tissue, and nerve tissue. It is possible that
cell division may always take place by the indirect method. |
|
|
The process of indirect cell division is characterized by a
series of complex changes in the nucleus, leading to its subdivision; this is
followed by cleavage of the cell protoplasm. Starting with the nucleus in the
quiescent or resting stage, these changes may be briefly grouped under
the four following phases (Fig.
2). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
Note |
|
The Ovum |
|
|
|
The ova are developed from the primitive germ cells which are imbedded
in the substance of the ovaries. Each primitive germ cell gives rise, by
repeated divisions, to a number of smaller cells termed oögonia, from
which the ova or primary oöcytes are developed. |
|
|
Human ova are extremely minute, measuring about |
|
|
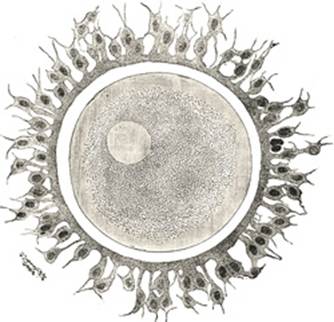
In appearance and structure the ovum (Fig. 3) differs little
from an ordinary cell, but distinctive names have been applied to its several
parts; thus, the cell substance is known as the yolk or oöplasm,
the nucleus as the germinal vesicle, and the nucleolus as the germinal
spot. The ovum is enclosed within a thick, transparent envelope, the zona
striata or zona pellucida, adhering to the outer surface of which
are several layers of cells, derived from those of the follicle and
collectively constituting the corona radiata. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Yolk.—The yolk comprises ( |
|
|
|
|
|
Germinal Vesicle.—The germinal
vesicle or nucleus is a large spherical body which at first occupies a nearly
central position, but becomes eccentric as the growth of the ovum proceeds.
Its structure is that of an ordinary cell-nucleus, viz., it consists of a
reticulum or karyomitome, the meshes of which are filled with karyoplasm,
while connected with, or imbedded in, the reticulum are a number of chromatin
masses or chromosomes, which may present the appearance of a skein or may
assume the form of rods or loops. The nucleus is enclosed by a delicate
nuclear membrane, and contains in its interior a well-defined nucleolus or germinal
spot. |
|
|
|
|
|
Coverings of the Ovum.—The zona
striata or zona pellucida (Fig. 3) is a thick
membrane, which, under the higher powers of the microscope, is seen to be
radially striated. It persists for some time after fertilization has
occurred, and may serve for protection during the earlier stages of
segmentation. It is not yet determined whether the zona striata is a product
of the cytoplasm of the ovum or of the cells of the corona radiata, or both. |
|
|
The corona radiata (Fig. 3) consists or two
or three strata of cells; they are derived from the cells of the follicle,
and adhere to the outer surface of the zona striata when the ovum is set free
from the follicle; the cells are radially arranged around the zona, those of
the innermost layer being columnar in shape. The cells of the corona radiata
soon disappear; in some animals they secrete, or are replaced by, a layer of
adhesive protein, which may assist in protecting and nourishing the ovum. |
|
|
The phenomena attending the discharge of the ova from the
follicles belong more to the ordinary functions of the ovary than to the
general subject of embryology, and are therefore described with the anatomy
of the ovaries. 4 |
|
|
|
|
|
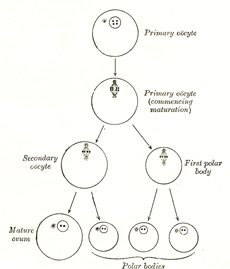
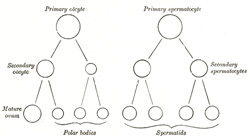
Maturation of the Ovum.—Before an
ovum can be fertilized it must undergo a process of maturation or ripening.
This takes place previous to or immediately after its escape from the
follicle, and consists essentially of an unequal subdivision of the ovum (Fig. 4) first into two
and then into four cells. Three of the four cells are small, incapable of
further development, and are termed polar bodies or polocytes,
while the fourth is large, and constitutes the mature ovum. The
process of maturation has not been observed in the human ovum, but has been
carefully studied in the ova of some of the lower animals, to which the
following description applies. |
|
|
It was pointed out on page |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
This second division is also unequal, producing a large cell
which constitutes the mature ovum, and a small cell, the second
polar body. The first polar body frequently divides while the second is
being formed, and as a final result four cells are produced, viz., the mature
ovum and three polar bodies, each of which contains two chromosomes, i.e.,
one-half the number present in the nuclei of the somatic cells of members of
the same species. The nucleus of the mature ovum is termed the female
pronucleus. |
|
The spermatozoa or male germ cells are developed in the
testes and are present in enormous numbers in the seminal fluid. Each
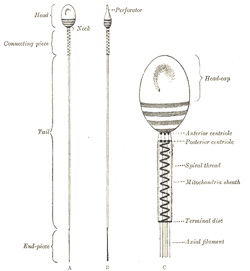
consists of a small but greatly modified cell. The human spermatozoön
possesses a head, a neck, a connecting piece or body,
and a tail (Fig. 6). |
|
|
|
|
|
FIG. |
|
|
|
|
|
The head is oval or elliptical, but flattened, so
that when viewed in profile it is pear-shaped. Its anterior two-thirds are
covered by a layer of modified protoplasm, which is named the head-cap.
This, in some animals, e. g., the salamander, is prolonged into a
barbed spear-like process or perforator, which probably facilitates
the entrance of the spermatozoön into the ovum. The posterior part of the
head exhibits an affinity for certain reagents, and presents a transversely
striated appearance, being crossed by three or four dark bands. In some
animals a central rodlike filament extends forward for about two-thirds of
the length of the head, while in others a rounded body is seen near its
center. The head contains a mass of chromatin, and is generally regarded as
the nucleus of the cell surrounded by a thin envelope. |
|
|
The neck is less constricted in the human
spermatozoön than in those of some of the lower animals. The anterior
centriole, represented by two or three rounded particles, is situated at
the junction of the head and neck, and behind it is a band of homogeneous
substance. |
|
|
The connecting piece or body is rod-like, and
is limited behind by a terminal disk. The posterior centriole
is placed at the junction of the body and neck and, like the anterior,
consists of two or three rounded particles. From this centriole an axial
filament, surrounded by a sheath, runs backward through the body and
tail. In the body the sheath of the axial filament is encircled by a spiral
thread, around which is an envelope containing mitochondria granules, and
termed the mitochondria sheath. |
|
|
The tail is of great length, and consists of the
axial thread or filament, surrounded by its sheath, which may contain a
spiral thread or may present a striated appearance. The terminal portion or end-piece
of the tail consists of the axial filament only. |
|
|
|
|
|
FIG. |
|
|
|
|
|
Krause gives the length of the human spermatozoön as between
|
|
|
By virtue of their tails, which act as propellers, the
spermatozoa are capable of free movement, and if placed in favorable
surroundings, e. g., in the female passages, will retain their
vitality and power of fertilizing for several days. In certain animals, e.
g., bats, it has been proved that spermatozoa retained in the female
passages for several months are capable of fertilizing. |
|
|
The spermatozoa are developed from the primitive germ cells
which have become imbedded in the testes, and the stages of their development
are very similar to those of the maturation of the ovum. The primary germ
cells undergo division and produce a number of cells termed spermatogonia,
and from these the primary spermatocytes are derived. Each primary
spermatocyte divides into two secondary spermatocytes, and each
secondary spermatocyte into two spermatids or young spermatozoa; from
this it will be seen that a primary spermatocyte gives rise to four
spermatozoa. On comparing this process with that of the maturation of the
ovum (Fig. 7) it will
be observed that the primary spermatocyte gives rise to two cells, the
secondary spermatocytes, and the primary oöcyte to two cells, the secondary
oöcyte and the first polar body. Again, the two secondary spermatocytes by
their subdivision give origin to four spermatozoa, and the secondary oöcyte
and first polar body to four cells, the mature ovum and three polar bodies.
In the development of the spermatozoa, as in the maturation of the ovum,
there is a reduction of the nuclear chromosomes to one-half of those present
in the primary spermatocyte. But here the similarity ends, for it must be
noted that the four spermatozoa are of equal size, and each is capable of
fertilizing a mature ovum, whereas the three polar bodies are not only very
much smaller than the mature ovum but are incapable of further development,
and may be regarded as abortive ova. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
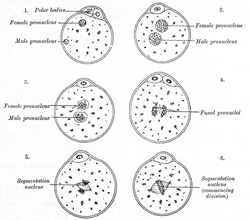
Fertilization consists in the union of the spermatozoön with the mature
ovum (Fig. 8). Nothing
is known regarding the fertilization of the human ovum, but the various
stages of the process have been studied in other mammals, and from the
knowledge so obtained it is believed that fertilization of the human ovum
takes place in the lateral or ampullary part of the uterine tube, and the
ovum is then conveyed along the tube to the cavity of the uterus—a journey
probably occupying seven or eight days and during which the ovum loses its corona
radiata and zona striata and undergoes segmentation. Sometimes the fertilized
ovum is arrested in the uterine tube, and there undergoes development, giving
rise to a tubal pregnancy; or it may fall into the abdominal cavity
and produce an abdominal pregnancy. Occasionally the ovum is not
expelled from the follicle when the latter ruptures, but is fertilized within
the follicle and produces what is known as an ovarian pregnancy. Under
normal conditions only one spermatozoön enters the yolk and takes part in the
process of fertilization. At the point where the spermatozoön is about to
pierce, the yolk is drawn out into a conical elevation, termed the cone of
attraction. As soon as the spermatozoön has entered the yolk, the
peripheral portion of the latter is transformed into a membrane, the vitelline
membrane which prevents the passage of additional spermatozoa.
Occasionally a second spermatozoön may enter the yolk, thus giving rise to a
condition of polyspermy: when this occurs the ovum usually develops in
an abnormal manner and gives rise to a monstrosity. Having pierced the yolk,
the spermatozoön loses its tail, while its head and connecting piece assume
the form of a nucleus containing a cluster of chromosomes. This constitutes
the male pronucleus, and associated with it there are a centriole and
centrosome. The male pronucleus passes more deeply into the yolk, and
coincidently with this the granules of the cytoplasm surrounding it become
radially arranged. The male and female pronuclei migrate toward each other,
and, meeting near the center of the yolk, fuse to form a new nucleus, the segmentation
nucleus, which therefore contains both male and female nuclear substance;
the former transmits the individualities of the male ancestors, the latter
those of the female ancestors, to the future embryo. By the union of the male
and female pronuclei the number of chromosomes is restored to that which is
present in the nuclei of the somatic cells. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
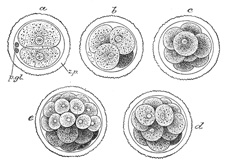
The early segmentation of the human ovum has not yet been observed, but
judging from what is known to occur in other mammals it may be regarded as
certain that the process starts immediately after the ovum has been
fertilized, i. e., while the ovum is in the uterine tube. The
segmentation nucleus exhibits the usual mitotic changes, and these are
succeeded by a division of the ovum into two cells of nearly equal size. 5
The process is repeated again and again, so that the two cells are succeeded
by four, eight, sixteen, thirty-two, and so on, with the result that a mass
of cells is found within the zona striata, and to this mass the term morula
is applied (Fig. 9).
The segmentation of the mammalian ovum may not take place in the regular
sequence of two, four, eight, etc., since one of the two first formed cells
may subdivide more rapidly than the other, giving rise to a three-or a
five-cell stage. The cells of the morula are at first closely aggregated, but
soon they become arranged into an outer or peripheral layer, the trophoblast,
which does not contribute to the formation of the embryo proper, and an inner
cell-mass, from which the embryo is developed. Fluid collects between the
trophoblast and the greater part of the inner cell-mass, and thus the morula
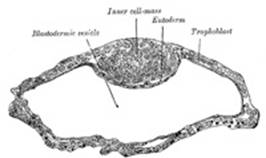
is converted into a vesicle, the blastodermic vesicle (Fig. 10). The inner
cell-mass remains in contact, however, with the trophoblast at one pole of the
ovum; this is named the embryonic pole, since it indicates the
situation where the future embryo will be developed. The cells of the
trophoblast become differentiated into two strata: an outer, termed the syncytium
or syncytiotrophoblast, so named because it consists of a layer of
protoplasm studded with nuclei, but showing no evidence of subdivision into
cells; and an inner layer, the cytotrophoblast or layer of
Langhans, in which the cell outlines are defined. As already stated, the
cells of the trophoblast do not contribute to the formation of the embryo
proper; they form the ectoderm of the chorion and play an important part in
the development of the placenta. On the deep surface of the inner cell-mass a
layer of flattened cells, the entoderm, is differentiated and quickly
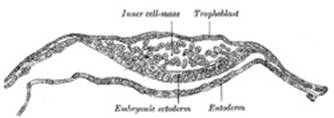
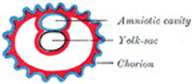
assumes the form of a small sac, the yolk-sac. Spaces appear between
the remaining cells of the mass (Fig. 11), and by the
enlargement and coalescence of these spaces a cavity, termed the amniotic
cavity (Fig. 12),
is gradually developed. The floor of this cavity is formed by the embryonic
disk composed of a layer of prismatic cells, the embryonic ectoderm,
derived from the inner cell-mass and lying in apposition with the entoderm. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
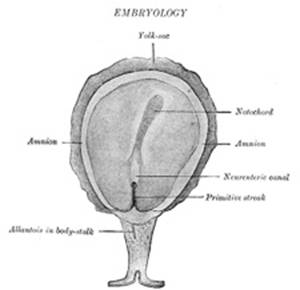
The Primitive Streak; Formation of the Mesoderm.—The embryonic disk becomes oval and then pear-shaped,
the wider end being directed forward. Near the narrow, posterior end an
opaque streak, the primitive streak (Figs. 13 and 14), makes its appearance
and extends along the middle of the disk for about one-half of its length; at
the anterior end of the streak there is a knob-like thickening termed Hensen’s
knot. A shallow groove, the primitive groove, appears on the
surface of the streak, and the anterior end of this groove communicates by
means of an aperture, the blastophore, with the yolk-sac. The
primitive streak is produced by a thickening of the axial part of the
ectoderm, the cells of which multiply, grow downward, and blend with those of
the subjacent entoderm (Fig.
15). From the sides of the primitive streak a third layer of cells, the mesoderm,
extends lateralward between the ectoderm and entoderm; the caudal end of the
primitive streak forms the cloacal membrane. |
|
|
|
|
|
FIG. |
|
|
|
|
|
The extension of the mesoderm takes place throughout the
whole of the embryonic and extra-embryonic areas of the ovum, except in
certain regions. One of these is seen immediately in front of the neural
tube. Here the mesoderm extends forward in the form of two crescentic masses,
which meet in the middle line so as to enclose behind them an area which is
devoid of mesoderm. Over this area the ectoderm and entoderm come into direct
contact with each other and constitute a thin membrane, the buccopharyngeal
membrane, which forms a septum between the primitive mouth and pharynx.
In front of the buccopharyngeal area, where the lateral crescents of mesoderm
fuse in the middle line, the pericardium is afterward developed, and this region
is therefore designated the pericardial area. A second region where
the mesoderm is absent, at least for a time, is that immediately in front of
the pericardial area. This is termed the proamniotic area, and is the
region where the proamnion is developed; in man, however, a proamnion
is apparently never formed. A third region is at the hind end of the embryo
where the ectoderm and entoderm come into apposition and form the cloacal
membrane. |
|
|
The blastoderm now consists of three layers, named from
without inward: ectoderm, mesoderm, and entoderm; each has distinctive
characteristics and gives rise to certain tissues of the body. 6 |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Ectoderm.—The ectoderm consists of columnar
cells, which are, however, somewhat flattened or cubical toward the margin of
the embryonic disk. It forms the whole of the nervous system, the epidermis
of the skin, the lining cells of the sebaceous, sudoriferous, and mammary
glands, the hairs and nails, the epithelium of the nose and adjacent air
sinuses, and that of the cheeks and roof of the mouth. From it also are
derived the enamel of the teeth, and the anterior lobe of the hypophysis
cerebri, the epithelium of the cornea, conjunctiva, and lacrimal glands, and
the neuro-epithelium of the sense organs. |
|
|
|
|
|
Entoderm.—The entoderm consists at first of
flattened cells, which subsequently become columnar. It forms the epithelial
lining of the whole of the digestive tube excepting part of the mouth and
pharynx and the terminal part of the rectum (which are lined by involutions
of the ectoderm), the lining cells of all the glands which open into the
digestive tube, including those of the liver and pancreas, the epithelium of
the auditory tube and tympanic cavity, of the trachea, bronchi, and air cells
of the lungs, of the urinary bladder and part of the urethra, and that which
lines the follicles of the thyroid gland and thymus. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Mesoderm.—The mesoderm consists of loosely
arranged branched cells surrounded by a considerable amount of intercellular
fluid. From it the remaining tissues of the body are developed. The
endothelial lining of the heart and blood-vessels and the blood corpuscles
are, however, regarded by some as being of entodermal origin. |
|
|
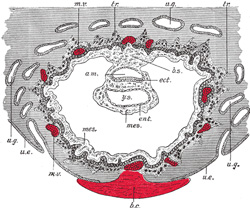
As the mesoderm develops between the ectoderm and entoderm
it is separated into lateral halves by the neural tube and notochord,
presently to be described. A longitudinal groove appears on the dorsal
surface of either half and divides it into a medial column, the paraxial
mesoderm, lying on the side of the neural tube, and a lateral portion,
the lateral mesoderm. The mesoderm in the floor of the groove connects
the paraxial with the lateral mesoderm and is known as the intermediate
cell-mass; in it the genito-urinary organs are developed. The lateral
mesoderm splits into two layers, an outer or somatic, which becomes
applied to the inner surface of the ectoderm, and with it forms the somatopleure;
and an inner or splanchnic, which adheres to the entoderm, and with it
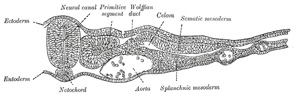
forms the splanchnopleure (Fig. 16). The space
between the two layers of the lateral mesoderm is termed the celom. |
|
|
Note |
|
Note |
||
|
|
||
|
|
||
|
|
||
|
FIG. |
||
|
|
||
|
In front of the primitive streak two longitudinal ridges, caused by a
folding up of the ectoderm, make their appearance, one on either side of the
middle line (Fig. 16).
These are named the neural folds; they commence some little distance
behind the anterior end of the embryonic disk, where they are continuous with
each other, and from there gradually extend backward, one on either side of
the anterior end of the primitive streak. Between these folds is a shallow
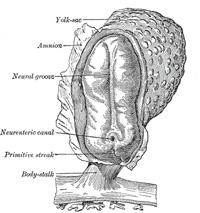
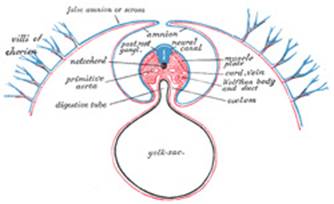
median groove, the neural groove (Figs. 16, 17). The groove gradually
deepens as the neural folds become elevated, and ultimately the folds meet
and coalesce in the middle line and convert the groove into a closed tube,
the neural tube or canal (Fig. 18), the ectodermal
wall of which forms the rudiment of the nervous system. After the coalescence
of the neural folds over the anterior end of the primitive streak, the
blastopore no longer opens on the surface but into the closed canal of the
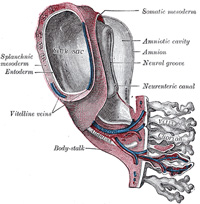
neural tube, and thus a transitory communication, the neurenteric canal,
is established between the neural tube and the primitive digestive tube. The
coalescence of the neural folds occurs first in the region of the hind-brain,
and from there extends forward and backward; toward the end of the third week
the front opening (anterior neuropore) of the tube finally closes at the
anterior end of the future brain, and forms a recess which is in contact, for
a time, with the overlying ectoderm; the hinder part of the neural groove
presents for a time a rhomboidal shape, and to this expanded portion the term
sinus rhomboidalis has been applied (Fig. 18). Before the
neural groove is closed a ridge of ectodermal cells appears along the
prominent margin of each neural fold; this is termed the neural crest
or ganglion ridge, and from it the spinal and cranial nerve ganglia
and the ganglia of the sympathetic nervous system are developed. By the upward
growth of the mesoderm the neural tube is ultimately separated from the
overlying ectoderm. |
|
|
|
|
|
FIG. |
|
|
|
|
|
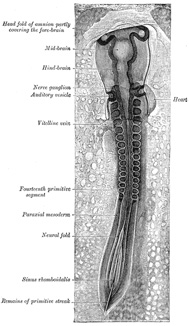
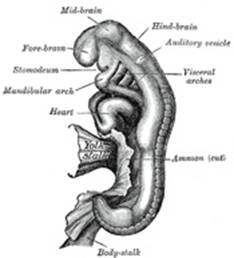
The cephalic end of the neural groove exhibits several
dilatations, which, when the tube is closed, assume the form of three
vesicles; these constitute the three primary cerebral vesicles, and
correspond respectively to the future fore-brain (prosencephalon),
mid-brain (mesencephalon), and hind-brain (rhombencephalon)
(Fig. 18). The walls
of the vesicles are developed into the nervous tissue and neuroglia of the
brain, and their cavities are modified to form its ventricles. The remainder
of the tube forms the medulla spinalis or spinal cord; from its
ectodermal wall the nervous and neuroglial elements of the medulla spinalis
are developed while the cavity persists as the central canal. |
|
|
|
|
|
|
|
|
The notochord (Fig.
19) consists of a rod of cells situated on the ventral aspect of the
neural tube; it constitutes the foundation of the axial skeleton, since
around it the segments of the vertebral column are formed. Its appearance
synchronizes with that of the neural tube. On the ventral aspect of the
neural groove an axial thickening of the entoderm takes place; this
thickening assumes the appearance of a furrow—the chordal furrow—the
margins of which come into contact, and so convert it into a solid rod of
cells—the notochord—which is then separated from the entoderm. It
extends throughout the entire length of the future vertebral column, and
reaches as far as the anterior end of the mid-brain, where it ends in a
hook-like extremity in the region of the future dorsum sellæ of the sphenoid
bone. It lies at first between the neural tube and the entoderm of the
yolk-sac, but soon becomes separated from them by the mesoderm, which grows
medial-ward and surrounds it. From the mesoderm surrounding the neural tube
and notochord, the skull and vertebral column, and the membranes of the brain
and medulla spinalis are developed. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
||
|
|
||
|
|
||
|
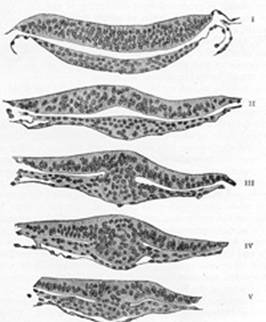
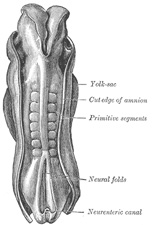
Toward the end of the second week transverse segmentation of the
paraxial mesoderm begins, and it is converted into a series of well-defined,
more or less cubical masses, the primitive segments (Figs. 18, 19, 20), which occupy the
entire length of the trunk on either side of the middle line from the
occipital region of the head. Each segment contains a central cavity—myocœl—which,
however, is soon filled with angular and spindle-shaped cells. |
|
|
|
|
|
FIG. |
|
|
|
|
|
The primitive segments lie immediately under the ectoderm on
the lateral aspect of the neural tube and notochord, and are connected to the
lateral mesoderm by the intermediate cell-mass. Those of the trunk may
be arranged in the following groups, viz.: cervical |
|
|
|
|
|
|
|
|
The embryo increases rapidly in size, but the circumference of the
embryonic disk, or line of meeting of the embryonic and amniotic parts of the
ectoderm, is of relatively slow growth and gradually comes to form a
constriction between the embryo and the greater part of the yolk-sac. By
means of this constriction, which corresponds to the future umbilicus, a
small part of the yolk-sac is enclosed within the embryo and constitutes the
primitive digestive tube. |
|
|
|
|
|
FIG. |
|
|
|
|
|
The embryo increases more rapidly in length than in width,
and its cephalic and caudal ends soon extend beyond the corresponding parts
of the circumference of the embryonic disk and are bent in a ventral
direction to form the cephalic and caudal folds respectively (Figs. 26 and 27). The cephalic fold is
first formed, and as the proamniotic area (page 47) lying immediately in
front of the pericardial area (page 47) forms the anterior limit of the
circumference of the embryonic disk, the forward growth of the head
necessarily carries with it the posterior end of the pericardial area, so
that this area and the buccopharyngeal membrane are folded back under the
head of the embryo which now encloses a diverticulum of the yolk-sac named
the fore-gut. The caudal end of the embryo is at first connected to
the chorion by a band of mesoderm called the body-stalk, but with the
formation of the caudal fold the body-stalk assumes a ventral position; a
diverticulum of the yolk-sac extends into the tail fold and is termed the hind-gut.
Between the fore-gut and the hind-gut there exists for a time a wide opening
into the yolk-sac, but the latter is gradually reduced to a small pear-shaped
sac (sometimes termed the umbilical vesicle), and the channel of
communication is at the same time narrowed and elongated to form a tube
called the vitelline duct. |
|
|
|
||
|
|
||
|
|
||
|
The yolk-sac (Figs.
22 and 23) is
situated on the ventral aspect of the embryo; it is lined by entoderm,
outside of which is a layer of mesoderm. It is filled with fluid, the vitelline
fluid, which possibly may be utilized for the nourishment of the embryo
during the earlier stages of its existence. Blood is conveyed to the wall of
the sac by the primitive aortæ, and after circulating through a wide-meshed
capillary plexus, is returned by the vitelline veins to the tubular heart of
the embryo. This constitutes the vitelline circulation, and by means
of it nutritive material is absorbed from the yolk-sac and conveyed to the
embryo. At the end of the fourth week the yolk-sac presents the appearance of
a small pear-shaped vesicle (umbilical vesicle) opening into the digestive
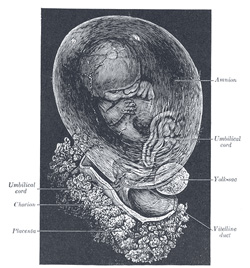
tube by a long narrow tube, the vitelline duct. The vesicle can be
seen in the after-birth as a small, somewhat oval-shaped body whose diameter
varies from 1 mm. to 5 mm.; it is situated between the amnion and the chorion
and may lie on or at a varying distance from the placenta. As a rule the duct
undergoes complete obliteration during the seventh week, but in about three
per cent. of cases its proximal part persists as a diverticulum from the
small intestine, Meckel’s diverticulum, which is situated about three
or four feet above the ileocolic junction, and may be attached by a fibrous
cord to the abdominal wall at the umbilicus. Sometimes a narrowing of the
lumen of the ileum is seen opposite the site of attachment of the duct. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
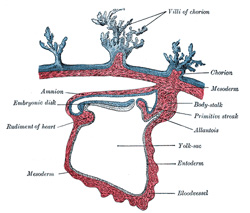
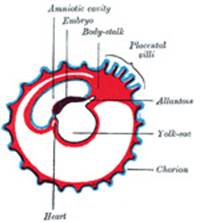
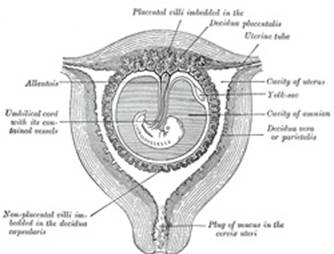
The Allantois (Figs.
25 to 28).—The allantois arises as a tubular diverticulum of the
posterior part of the yolk-sac; when the hind-gut is developed the allantois
is carried backward with it and then opens into the cloaca or terminal part
of the hind-gut: it grows out into the body-stalk, a mass of mesoderm which
lies below and around the tail end of the embryo. The diverticulum is lined
by entoderm and covered by mesoderm, and in the latter are carried the
allantoic or umbilical vessels. |
|
|
In reptiles, birds, and many mammals the allantois becomes expanded into
a vesicle which projects into the extra-embryonic celom. If its further
development be traced in the bird, it is seen to project to the right side of
the embryo, and, gradually expanding, it spreads over its dorsal surface as a
flattened sac between the amnion and the serosa, and extending in all directions,
ultimately surrounds the yolk. Its outer wall becomes applied to and fuses
with the serosa, which lies immediately inside the shell membrane. Blood is
carried to the allantoic sac by the two allantoic or umbilical arteries,
which are continuous with the primitive aortæ, and after circulating through
the allantoic capillaries, is returned to the primitive heart by the two
umbilical veins. In this way the allantoic circulation, which is of the
utmost importance in connection with the respiration and nutrition of the
chick, is established. Oxygen is taken from, and carbonic acid is given up to
the atmosphere through the egg-shell, while nutritive materials are at the
same time absorbed by the blood from the yolk. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
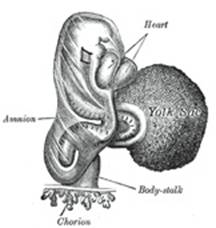
In man and other primates the nature of the allantois is
entirely different from that just described. Here it exists merely as a
narrow, tubular diverticulum of the hind-gut, and never assumes the form of a
vesicle outside the embryo. With the formation of the amnion the embryo is,
in most animals, entirely separated from the chorion, and is only again
united to it when the allantoic mesoderm spreads over and becomes applied to
its inner surface. The human embryo, on the other hand, as was pointed out by
His, is never wholly separated from the chorion, its tail end being from the
first connected with the chorion by means of a thick band of mesoderm, named
the body-stalk (Bauchstiel); into this stalk the tube of the allantois
extends (Fig. 21). |
|
|
|
|
|
The Amnion.—The amnion is
a membranous sac which surrounds and protects the embryo. It is developed in
reptiles, birds, and mammals, which are hence called “Amniota;” but not in
amphibia and fishes, which are consequently termed “Anamnia.” |
|
|
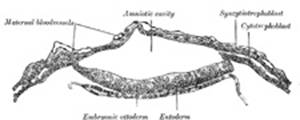
In the human embryo the earliest stages of the formation of
the amnion have not been observed; in the youngest embryo which has been
studied the amnion was already present as a closed sac (Figs. 24 and 32), and, as indicated on
page 46, appears in the inner cell-mass as a cavity. This cavity is roofed in
by a single stratum of flattened, ectodermal cells, the amniotic ectoderm,
and its floor consists of the prismatic ectoderm of the embryonic disk—the
continuity between the roof and floor being established at the margin of the
embryonic disk. Outside the amniotic ectoderm is a thin layer of mesoderm, which
is continuous with that of the somatopleure and is connected by the
body-stalk with the mesodermal lining of the chorion. |
|
|
|
|
|
FIG. |
|
|
|
|
|
When first formed the amnion is in contact with the body of
the embryo, but about the fourth or fifth week fluid (liquor amnii)
begins to accumulate within it. This fluid increases in quantity and causes
the amnion to expand and ultimately to adhere to the inner surface of the
chorion, so that the extra-embryonic part of the celom is obliterated. The
liquor amnii increases in quantity up to the sixth or seventh month of
pregnancy, after which it diminishes somewhat; at the end of pregnancy it
amounts to about |
|
|
In reptiles, birds, and many mammals the amnion is developed
in the following manner: At the point of constriction where the primitive
digestive tube of the embryo joins the yolk-sac a reflection or folding
upward of the somatopleure takes place. This, the amniotic fold (Fig. 29), first makes
its appearance at the cephalic extremity, and subsequently at the caudal end
and sides of the embryo, and gradually rising more and more, its different
parts meet and fuse over the dorsal aspect of the embryo, and enclose a
cavity, the amniotic cavity. After the fusion of the edges of the
amniotic fold, the two layers of the fold become completely separated, the
inner forming the amnion, the outer the false amnion or serosa.
The space between the amnion and the serosa constitutes the extra-embryonic
celom, and for a time communicates with the embryonic celom. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
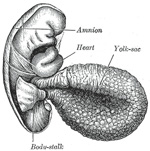
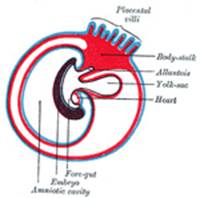
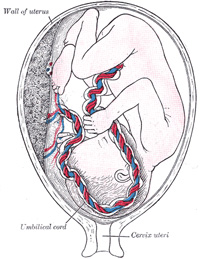
The Umbilical Cord and Body-stalk.—The umbilical cord (Fig.
28) attaches the fetus to the placenta; its length at full time, as a
rule, is about equal to the length of the fetus, i.e., about 50 cm.,
but it may be greatly diminished or increased. The rudiment of the umbilical
cord is represented by the tissue which connects the rapidly growing embryo
with the extra-embryonic area of the ovum. Included in this tissue are the
body-stalk and the vitelline duct—the former containing the allantoic
diverticulum and the umbilical vessels, the latter forming the communication
between the digestive tube and the yolk-sac. The body-stalk is the posterior
segment of the embryonic area, and is attached to the chorion. It consists of
a plate of mesoderm covered by thickened ectoderm on which a trace of the
neural groove can be seen, indicating its continuity with the embryo. Running
through its mesoderm are the two umbilical arteries and the two umbilical
veins, together with the canal of the allantois—the last being lined by
entoderm (Fig. 31).
Its dorsal surface is covered by the amnion, while its ventral surface is
bounded by the extra-embryonic celom, and is in contact with the vitelline
duct and yolk-sac. With the rapid elongation of the embryo and the formation
of the tail fold, the body stalk comes to lie on the ventral surface of the
embryo (Figs. 27 and 28), where its mesoderm
blends with that of the yolk-sac and the vitelline duct. The lateral leaves
of somatopleure then grow round on each side, and, meeting on the ventral
aspect of the allantois, enclose the vitelline duct and vessels, together
with a part of the extra-embryonic celom; the latter is ultimately
obliterated. The cord is covered by a layer of ectoderm which is continuous
with that of the amnion, and its various constitutents are enveloped by embryonic
gelatinous tissue, jelly of Wharton. The vitelline vessels and duct,
together with the right umbilical vein, undergo atrophy and disappear; and
thus the cord, at birth, contains a pair of umbilical arteries and one (the
left) umbilical vein. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Implantation or Imbedding of the Ovum.—As described (page |
|
|
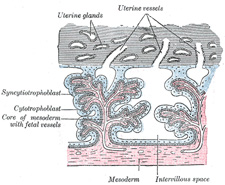
The structure actively concerned in the process of
excavation is the trophoblast of the ovum, which possesses the power of
dissolving and absorbing the uterine tissues. The trophoblast proliferates
rapidly and forms a network of branching processes which cover the entire
ovum and invade and destroy the maternal tissues and open into the maternal
bloodvessels, with the result that the spaces in the trophoblastic network
are filled with maternal blood; these spaces communicate freely with one
another and become greatly distended and form the intervillous space. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
The Decidua.—Before the
fertilized ovum reaches the uterus, the mucous membrane of the body of the
uterus undergoes important changes and is then known as the decidua.
The thickness and vascularity of the mucous membrane are greatly increased;
its glands are elongated and open on its free surface by funnel-shaped
orifices, while their deeper portions are tortuous and dilated into irregular
spaces. The interglandular tissue is also increased in quantity, and is
crowded with large round, oval, or polygonal cells, termed decidual cells.
These changes are well advanced by the second month of pregnancy, when the
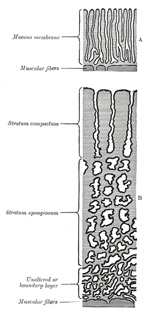
mucous membrane consists of the following strata (Fig. 33): (1) stratum
compactum, next the free surface; in this the uterine glands are only slightly
expanded, and are lined by columnar cells; (2) stratum spongiosum, in
which the gland tubes are greatly dilated and very tortuous, and are
ultimately separated from one another by only a small amount of
interglandular tissue, while their lining cells are flattened or cubical; (3)
a thin unaltered or boundary layer, next the uterine muscular
fibers, containing the deepest parts of the uterine glands, which are not
dilated, and are lined with columnar epithelium; it is from this epithelium
that the epithelial lining of the uterus is regenerated after pregnancy.
Distinctive names are applied to different portions of the decidua. The part
which covers in the ovum is named the decidua capsularis; the portion
which intervenes between the ovum and the uterine wall is named the decidua
basalis or decidua placentalis; it is here that the placenta is
subsequently developed. The part of the decidua which lines the remainder of
the body of the uterus is known as the decidua vera or decidua
parietalis. |
|
|
Coincidently with the growth of the embryo, the decidua
capsularis is thinned and extended (Fig. 34) and the space
between it and the decidua vera is gradually obliterated, so that by the
third month of pregnancy the two are in contact. By the fifth month of
pregnancy the decidua capsularis has practically disappeared, while during
the succeeding months the decidua vera also undergoes atrophy, owing to the
increased pressure. The glands of the stratum compactum are obliterated, and
their epithelium is lost. In the stratum spongiosum the glands are compressed
and appear as slit-like fissures, while their epithelium undergoes
degeneration. In the unaltered or boundary layer, however, the glandular
epithelium retains a columnar or cubical form. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
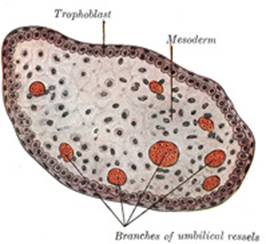
The Chorion (Figs. 23
to28).—The chorion consists of two layers: an outer formed by
the primitive ectoderm or trophoblast, and an inner by the somatic mesoderm;
with this latter the amnion is in contact. The trophoblast is made up of an
internal layer of cubical or prismatic cells, the cytotrophoblast or layer
of Langhans, and an external layer of richly nucleated protoplasm devoid
of cell boundaries, the syncytiotrophoblast. It undergoes rapid
proliferation and forms numerous processes, the chorionic villi, which
invade and destroy the uterine decidua and at the same time absorb from it
nutritive materials for the growth of the embryo. The chorionic villi are at
first small and non-vascular, and consist of trophoblast only, but they
increase in size and ramify, while the mesoderm, carrying branches of the
umbilical vessels, grows into them, and in this way they are vascularized.
Blood is carried to the villi by the branches of the umbilical arteries, and
after circulating through the capillaries of the villi, is returned to the
embryo by the umbilical veins. Until about the end of the second month of
pregnancy the villi cover the entire chorion, and are almost uniform in size (Fig. 25), but after this
they develop unequally. The greater part of the chorion is in contact with
the decidua capsularis (Fig.
34), and over this portion the villi, with their contained vessels,
undergo atrophy, so that by the fourth month scarcely a trace of them is
left, and hence this part of the chorion becomes smooth, and is named the chorion
læve; as it takes no share in the formation of the placenta, it is also
named the non-placental part of the chorion. On the other hand, the villi on
that part of the chorion which is in contact with the decidua placentalis
increase greatly in size and complexity, and hence this part is named the chorion
frondosum (Fig. 28). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
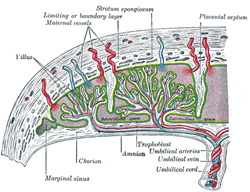
The Placenta.—The placenta
connects the fetus to the uterine wall, and is the organ by means of which
the nutritive, respiratory, and excretory functions of the fetus are carried
on. It is composed of fetal and maternal portions. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
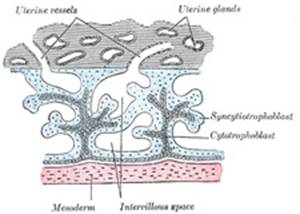
Fetal Portion.—The fetal
portion of the placenta consists of the villi of the chorion frondosum; these
branch repeatedly, and increase enormously in size. These greatly ramified
villi are suspended in the intervillous space, and are bathed in maternal
blood, which is conveyed to the space by the uterine arteries and carried
away by the uterine veins. A branch of an umbilical artery enters each villus
and ends in a capillary plexus from which the blood is drained by a tributary
of the umbilical vein. The vessels of the villus are surrounded by a thin
layer of mesoderm consisting of gelatinous connective tissue, which is
covered by two strata of ectodermal cells derived from the trophoblast: the
deeper stratum, next the mesodermic tissue, represents the cytotrophoblast or
layer of Langhans; the superficial, in contact with the maternal blood, the
syncytiotrophoblast (Figs.
36 and 37). After
the fifth month the two strata of cells are replaced by a single layer of
somewhat flattened cells. |
|
|
|
|
|
Maternal Portion.—The maternal
portion of the placenta is formed by the decidua placentalis containing the
intervillous space. As already explained, this space is produced by the
enlargement and intercommunication of the spaces in the trophoblastic
network. The changes involve the disappearance of the greater portion of the
stratum compactum, but the deeper part of this layer persists and is
condensed to form what is known as the basal plate. Between this plate
and the uterine muscular fibres are the stratum spongiosum and the boundary
layer; through these and the basal plate the uterine arteries and veins pass
to and from the intervillous space. The endothelial lining of the uterine
vessels ceases at the point where they terminate in the intervillous space
which is lined by the syncytiotrophoblast. Portions of the stratum compactum
persist and are condensed to form a series of septa, which extend from the
basal plate through the thickness of the placenta and subdivide it into the
lobules or cotyledons seen on the uterine surface of the detached placenta. |
|
|
|
|
|
FIG. |
|
|
|
|
|
The fetal and maternal blood currents traverse the placenta,
the former passing through the bloodvessels of the placental villi and the
latter through the intervillous space (Fig. 39). The two
currents do not intermingle, being separated from each other by the delicate
walls of the villi. Nevertheless, the fetal blood is able to absorb, through
the walls of the villi, oxygen and nutritive materials from the maternal
blood, and give up to the latter its waste products. The blood, so purified,
is carried back to the fetus by the umbilical vein. It will thus be seen that
the placenta not only establishes a mechanical connection between the mother
and the fetus, but subserves for the latter the purposes of nutrition,
respiration, and excretion. In favor of the view that the placenta possesses
certain selective powers may be mentioned the fact that glucose is more
plentiful in the maternal than in the fetal blood. It is interesting to note
also that the proportion of iron, and of lime and potash, in the fetus is
increased during the last months of pregnancy. Further, there is evidence
that the maternal leucocytes may migrate into the fetal blood, since
leucocytes are much more numerous in the blood of the umbilical vein than in
that of the umbilical arteries. |
|
|
The placenta is usually attached near the fundus uteri, and
more frequently on the posterior than on the anterior wall of the uterus. It
may, however, occupy a lower position and, in rare cases, its site is close
to the orificium internum uteri, which it may occlude, thus giving rise to
the condition known as placenta previa. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Separation of the Placenta.—After the
child is born, the placenta and membranes are expelled from the uterus as the
after-birth. The separation of the placenta from the uterine wall
takes place through the stratum spongiosum, and necessarily causes rupture of
the uterine vessels. The orifices of the torn vessels are, however, closed by
the firm contraction of the uterine muscular fibers, and thus postpartum
hemorrhage is controlled. The epithelial lining of the uterus is
regenerated by the proliferation and extension of the epithelium which lines
the persistent portions of the uterine glands in the unaltered layer of the
decidua. |
|
|
The expelled placenta appears as a discoid mass which weighs
about |
|
|
On section, the placenta presents a soft, spongy appearance,
caused by the greatly branched villi; surrounding them is a varying amount of
maternal blood giving the dark red color to the placenta. Many of the larger
villi extend from the chorionic to the decidual surface, while others are
attached to the septa which separate the cotyledons; but the great majority
of the villi hang free in the intervillous space. |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
Note |
|
|
|||
|
|
|||
|
|
|||
|
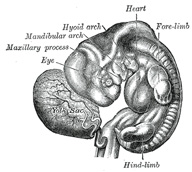
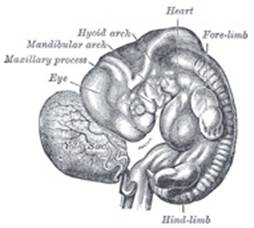
The Branchial or Visceral Arches and Pharyngeal Pouches.—In the lateral walls of the anterior part of the
fore-gut five pharyngeal pouches appear (Fig. 42); each of the
upper four pouches is prolonged into a dorsal and a ventral diverticulum.
Over these pouches corresponding indentations of the ectoderm occur, forming
what are known as the branchial or outer pharyngeal grooves.
The intervening mesoderm is pressed aside and the ectoderm comes for a time
into contact with the entodermal lining of the fore-gut, and the two layers
unite along the floors of the grooves to form thin closing membranes
between the fore-gut and the exterior. Later the mesoderm again penetrates
between the entoderm and the ectoderm. In gill-bearing animals the closing
membranes disappear, and the grooves become complete clefts, the gill-clefts,
opening from the pharynx on to the exterior; perforation, however, does not
occur in birds or mammals. The grooves separate a series of rounded bars or
arches, the branchial or visceral arches, in which thickening
of the mesoderm takes place (Figs. 40 and 41). The dorsal ends of
these arches are attached to the sides of the head, while the ventral
extremities ultimately meet in the middle line of the neck. In all, six
arches make their appearance, but of these only the first four are visible
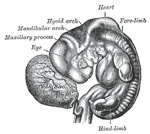
externally. The first arch is named the mandibular, and the second the hyoid;
the others have no distinctive names. In each arch a cartilaginous bar,
consisting of right and left halves, is developed, and with each of these
there is one of the primitive aortic arches. |
|
||
|
|
|
||
|
FIG. |
|
||
|
|
|
||
|
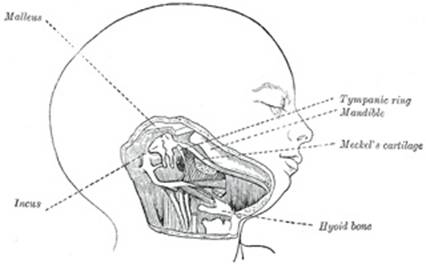
The mandibular arch lies between the first branchial groove and
the stomodeum; from it are developed the lower lip, the mandible, the muscles
of mastication, and the anterior part of the tongue. Its cartilaginous bar is
formed by what are known as Meckel’s cartilages (right and left) (Fig. 43); above this the
incus is developed. The dorsal end of each cartilage is connected with the
ear-capsule and is ossified to form the malleus; the ventral ends meet each
other in the region of the symphysis menti, and are usually regarded as
undergoing ossification to form that portion of the mandible which contains
the incisor teeth. The intervening part of the cartilage disappears; the
portion immediately adjacent to the malleus is replaced by fibrous membrane,
which constitutes the spheno-mandibular ligament, while from the connective
tissue covering the remainder of the cartilage the greater part of the
mandible is ossified. From the dorsal ends of the mandibular arch a
triangular process, the maxillary process, grows forward on either
side and forms the cheek and lateral part of the upper lip. The second
or hyoid arch assists in forming the side and front of the neck. From
its cartilage are developed the styloid process, stylohyoid ligament, and
lesser cornu of the hyoid bone. The stages probably arises in the upper part
of this arch. The cartilage of the third arch gives origin to the
greater cornu of the hyoid bone. The ventral ends of the second and third
arches unite with those of the opposite side, and form a transverse band,
from which the body of the hyoid bone and the posterior part of the tongue
are developed. The ventral portions of the cartilages of the fourth
and fifth arches unite to form the thyroid cartilage; from the
cartilages of the sixth arch the cricoid and arytenoid cartilages and
the cartilages of the trachea are developed. The mandibular and hyoid arches
grow more rapidly than those behind them, with the result that the latter
become, to a certain extent, telescoped within the former, and a deep
depression, the sinus cervicalis, is formed on either side of the
neck. This sinus is bounded in front by the hyoid arch, and behind by the
thoracic wall; it is ultimately obliterated by the fusion of its walls. |
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
From the first branchial groove the concha auriculæ and
external acoustic meatus are developed, while around the groove there appear,
on the mandibular and hyoid arches, a number of swellings from which the
auricula or pinna is formed. The first pharyngeal pouch is prolonged dorsally
to form the auditory tube and the tympanic cavity; the closing membrane
between the mandibular and hyoid arches is invaded by mesoderm, and forms the
tympanic membrane. No traces of the second, third, and fourth branchial
grooves persist. The inner part of the second pharyngeal pouch is named the sinus
tonsillaris; in it the tonsil is developed, above which a trace of the
sinus persists as the supratonsillar fossa. The fossa of Rosenmüller or
lateral recess of the pharynx is by some regarded as a persistent part of the
second pharyngeal pouch, but it is probably developed as a secondary
formation. From the third pharyngeal pouch the thymus arises as an entodermal
diverticulum on either side, and from the fourth pouches small diverticula
project and become incorporated with the thymus, but in man these diverticula
probably never form true thymus tissue. The parathyroids also arise as
diverticula from the third and fourth pouches. From the fifth pouches the
ultimobranchial bodies originate and are enveloped by the lateral
prolongations of the median thyroid rudiment; they do not, however, form true
thyroid tissue, nor are any traces of them found in the human adult. |
|
|
|
|
|
|
|
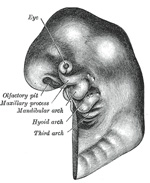
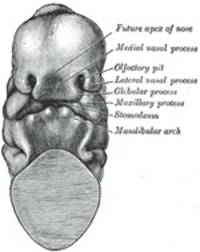
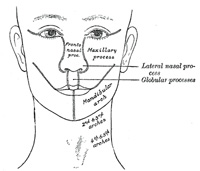
The Nose and Face.—During the
third week two areas of thickened ectoderm, the olfactory areas,
appear immediately under the fore-brain in the anterior wall of the
stomodeum, one on either side of a region termed the fronto-nasal process
(Fig. 44). By the
upgrowth of the surrounding parts these areas are converted into pits, the olfactory
pits, which indent the fronto-nasal process and divide it into a medial
and two lateral nasal processes (Fig. 45). The rounded
lateral angles of the medial process constitute the globular processes
of His. The olfactory pits form the rudiments of the nasal cavities, and from
their ectodermal lining the epithelium of the nasal cavities, with the
exception of that of the inferior meatuses, is derived. The globular
processes are prolonged backward as plates, termed the nasal laminæ:
these laminæ are at first some distance apart, but, gradually approaching,
they ultimately fuse and form the nasal septum; the processes themselves meet
in the middle line, and form the premaxillæ and the philtrum or central part
of the upper lip (Fig. 48).
The depressed part of the medial nasal process between the globular processes
forms the lower part of the nasal septum or columella; while above
this is seen a prominent angle, which becomes the future apex (Figs. 45, 46), and still higher a
flat area, the future bridge, of the nose. The lateral nasal processes form
the alæ of the nose. |
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
Continuous with the dorsal end of the mandibular arch, and
growing forward from its cephalic border, is a triangular process, the maxillary
process, the ventral extremity of which is separated from the mandibular
arch by a > shaped notch (Fig.
44). The maxillary process forms the lateral wall and floor of the orbit,
and in it are ossified the zygomatic bone and the greater part of the
maxilla; it meets with the lateral nasal process, from which, however, it is
separated for a time by a groove, the naso-optic furrow, that extends
from the furrow encircling the eyeball to the olfactory pit. The maxillary
processes ultimately fuse with the lateral nasal and globular processes, and
form the lateral parts of the upper lip and the posterior boundaries of the
nares (Figs. 47, 48). From the third to
the fifth month the nares are filled by masses of epithelium, on the breaking
down and disappearance of which the permanent openings are produced. The
maxillary process also gives rise to the lower portion of the lateral wall of
the nasal cavity. The roof of the nose and the remaining parts of the lateral
wall, viz., the ethmoidal labyrinth, the inferior nasal concha, the lateral
cartilage, and the lateral crus of the alar cartilage, are developed in the
lateral nasal process. By the fusion of the maxillary and nasal processes in
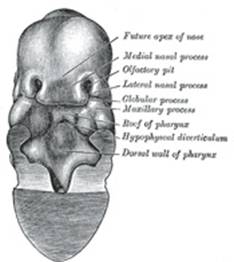
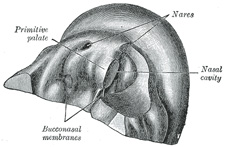
the roof of the stomodeum the primitive palate (Fig. 49) is formed, and
the olfactory pits extend backward above it. The posterior end of each pit is
closed by an epithelial membrane, the bucco-nasal membrane, formed by
the apposition of the nasal and stomodeal epithelium. By the rupture of these
membranes the primitive choanæ or openings between the olfactory pits
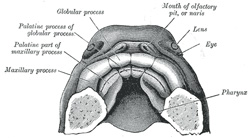
and the stomodeum are established. The floor of the nasal cavity is completed
by the development of a pair of shelf-like palatine processes which
extend medial-ward from the maxillary processes (Figs. 50 and 51); these coalesce with
each other in the middle line, and constitute the entire palate, except a
small part in front which is formed by the premaxillary bones. Two apertures
persist for a time between the palatine processes and the premaxillæ and
represent the permanent channels which in the lower animals connect the nose
and mouth. The union of the parts which form the palate commences in front,
the premaxillary and palatine processes joining in the eighth week, while the
region of the future hard palate is completed by the ninth, and that of the
soft palate by the eleventh week. By the completion of the palate the permanent
choanæ are formed and are situated a considerable distance behind the
primitive choanæ. The deformity known as cleft palate results from a
non-union of the palatine processes, and that of harelip through a non-union
of the maxillary and globular processes (see page 199). The nasal cavity
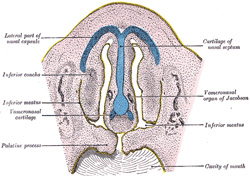
becomes divided by a vertical septum, which extends downward and backward
from the medial nasal process and nasal laminæ, and unites below with the
palatine processes. Into this septum a plate of cartilage extends from the
under aspect of the ethmoid plate of the chodrocranium. The anterior part of
this cartilaginous plate persists as the septal cartilage of the nose and the
medial crus of the alar cartilage, but the posterior and upper parts are
replaced by the vomer and perpendicular plate of the ethmoid. On either side
of the nasal septum, at its lower and anterior part, the ectoderm is
invaginated to form a blind pouch or diverticulum, which extends backward and
upward into the nasal septum and is supported by a curved plate of cartilage.
These pouches form the rudiments of the vomero-nasal organs of
Jacobson, which open below, close to the junction of the premaxillary and
maxillary bones. |
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
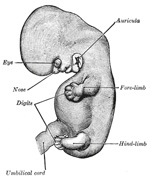
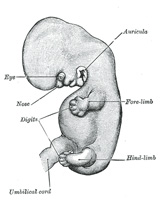
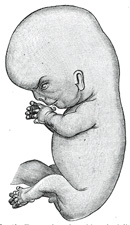
The Limbs.—The limbs
begin to make their appearance in the third week as small elevations or buds
at the side of the trunk (Fig.
52). Prolongations from the muscle- and cutis-plates of several primitive
segments extend into each bud, and carry with them the anterior divisions of
the corresponding spinal nerves. The nerves supplying the limbs indicate the
number of primitive segments which contribute to their formation—the upper
limb being derived from seven, viz., fourth cervical to second thoracic
inclusive, and the lower limb from ten, viz., twelfth thoracic to fourth
sacral inclusive. The axial part of the mesoderm of the limb-bud becomes
condensed and converted into its cartilaginous skeleton, and by the
ossification of this the bones of the limbs are formed. By the sixth week the
three chief divisions of the limbs are marked off by furrows—the upper into
arm, forearm, and hand; the lower into thigh, leg, and foot (Fig. 53). The limbs are
at first directed backward nearly parallel to the long axis of the trunk, and
each presents two surfaces and two borders. Of the surfaces, one—the future flexor
surface of the limb—is directed ventrally; the other, the extensor
surface, dorsally; one border, the preaxial, looks forward toward the
cephalic end of the embryo, and the other, the postaxial, backward
toward the caudal end. The lateral epicondyle of the humerus, the radius, and
the thumb lie along the preaxial border of the upper limb; and the medial
epicondyle of the femur, the tibia, and the great toe along the corresponding
border of the lower limb. The preaxial part is derived from the anterior
segments, the postaxial from the posterior segments of the limb-bud; and this
explains, to a large extent, the innervation of the adult limb, the nerves of
the more anterior segments being distributed along the preaxial (radial or
tibial), and those of the more posterior along the postaxial (ulnar or
fibular) border of the limb. The limbs next undergo a rotation or torsion
through an angle of 90° around their long axes the rotation being effected
almost entirely at the limb girdles. In the upper limb the rotation is
outward and forward; in the lower limb, inward and backward. As a consequence
of this rotation the preaxial (radial) border of the fore-limb is directed
lateralward, and the preaxial (tibial) border of the hind-limb is directed
medialward; thus the flexor surface of the fore-limb is turned forward, and
that of the hind-limb backward. |
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
||
|
|
||
|
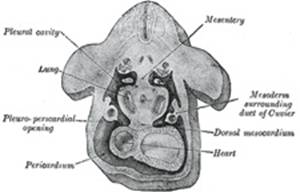
In the human embryo described by Peters the mesoderm outside the
embryonic disk is split into two layers enclosing an extra-embryonic cœlom;
there is no trace of an intra-embryonic cœlom. At a later stage four cavities
are formed within the embryo, viz., one on either side within the mesoderm of
the pericardial area, and one in either lateral mass of the general mesoderm.
All these are at first independent of each other and of the extra-embryonic
celom, but later they become continuous. The two cavities in the general
mesoderm unite on the ventral aspect of the gut and form the pleuro-peritoneal
cavity, which becomes continuous with the remains of the extra-embryonic
celom around the umbilicus; the two cavities in the pericardial area rapidly
join to form a single pericardial cavity, and this from two lateral
diverticula extend caudalward to open into the pleuro-peritoneal cavity (Fig. 54). |
|
|
|
|
|
FIG. |
|
|
|
|
|
Between the two latter diverticula is a mass of mesoderm
containing the ducts of Cuvier, and this is continuous ventrally with the
mesoderm in which the umbilical veins are passing to the sinus venosus. A
septum of mesoderm thus extends across the body of the embryo. It is attached
in front to the body-wall between the pericardium and umbilicus; behind to
the body-wall at the level of the second cervical segment; laterally it is
deficient where the pericardial and pleuro-peritoneal cavities communicate,
while it is perforated in the middle line by the foregut. This partition is
termed the septum transversum, and is at first a bulky plate of
tissue. As development proceeds the dorsal end of the septum is carried
gradually caudalward, and when it reaches the fifth cervical segment muscular
tissue with the phrenic nerve grows into it. It continues to recede, however,
until it reaches the position of the adult diaphragm on the bodies of the
upper lumbar vertebræ. The liver buds grow into the septum transversum and
undergo development there. |
|
|
The lung buds meantime have grown out from the fore-gut, and
project laterally into the forepart of the pleuro-peritoneal cavity; the
developing stomach and liver are imbedded in the septum transversum; caudal
to this the intestines project into the back part of the pleuro-peritoneal
cavity (Fig. 55).
Owing to the descent of the dorsal end of the septum transversum the
lung buds come to lie above the septum and thus pleural and peritoneal
portions of the pleuro-peritoneal cavity (still, however, in free
communication with one another) may be recognized; the pericardial cavity
opens into the pleural part. |
|
|
|
|
|
FIG. |
|
|
|
|
|
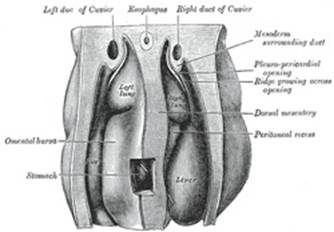
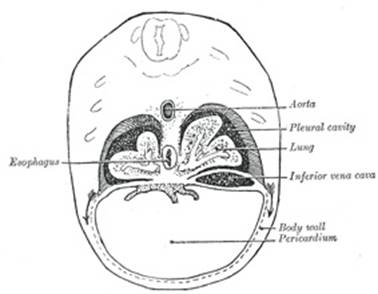
The ultimate separation of the permanent cavities from one
another is effected by the growth of a ridge of tissue on either side from
the mesoderm surrounding the duct of Cuvier (Figs. 54, 55). The front part of
this ridge grows across and obliterates the pleuro-pericardial opening; the
hinder part grows across the pleuro-peritoneal opening. |
|
|
|
|
|
FIG. |
|
|
|
|
|
With the continued growth of the lungs the pleural cavities
are pushed forward in the body-wall toward the ventral median line, thus
separating the pericardium from the lateral thoracic walls (Fig. 53). The further
development of the peritoneal cavity has been described with the development
of the digestive tube (page 168 et seq.). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
First Week.—During this
period the ovum is in the uterine tube. Having been fertilized in the upper
part of the tube, it slowly passes down, undergoing segmentation, and reaches
the uterus. Peters 9 described a specimen,
the age of which he reckoned as from three to four days. It was imbedded in
the decidua on the posterior wall of the uterus and enveloped by a decidua
capsularis, the central part of which, however, consisted merely of a layer
of fibrin. The ovum was in the form of a sac, the outer wall of which
consisted of a layer of trophoblast; inside this was a thin layer of mesoderm
composed of round, oval, and spindle-shaped cells. Numerous villous
processes—some consisting of trophoblast only, others possessing a core of
mesoderm—projected from the surface of the ovum into the surrounding decidua.
Inside this sac the rudiment of the embryo was found in the form of a patch
of ectoderm, covered by a small but completely closed amnion. It possessed a
minute yolk-sac and was surrounded by mesoderm, which was connected by a band
to that lining the trophoblast (Fig. 32). 10 |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Second Week.—By the end of
this week the ovum has increased considerably in size, and the majority of
its villi are vascularized. The embryo has assumed a definite form, and its
cephalic and caudal extremities are easily distinguished. The neural folds
are partly united. The embryo is more completely separated from the yolk-sac,
and the paraxial mesoderm is being divided into the primitive segments (Fig. 58). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Third Week.—By the end of
the third week the embryo is strongly curved, and the primitive segments
number about thirty. The primary divisions of the brain are visible, and the
optic and auditory vesicles are formed. Four branchial grooves are present:
the stomodeum is well-marked, and the bucco-pharyngeal membrane has
disappeared. The rudiments of the limbs are seen as short buds, and the Wolffian
bodies are visible (Fig.
59). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Fourth Week.—The embryo is
markedly curved on itself, and when viewed in profile is almost circular in
outline. The cerebral hemispheres appear as hollow buds, and the elevations
which form the rudiments of the auricula are visible. The limbs now appear as
oval flattened projections (Fig.
60). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Fifth Week.—The embryo is
less curved and the head is relatively of large size. Differentiation of the
limbs into their segments occurs. The nose forms a short, flattened
projection. The cloacal tubercle is evident (Fig. 61). |
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
FIG. |
|
|
|
|
|
|
|
|
Sixth Week.—The curvature
of the embryo is further diminished. The branchial grooves—except the
first—have disappeared, and the rudiments of the fingers and toes can be
recognized (Fig. 62). |
|
|
|
|
|
Seventh and Eighth Weeks.—The
flexure of the head is gradually reduced and the neck is somewhat lengthened.
The upper lip is completed and the nose is more prominent. The nostrils are
directed forward and the palate is not completely developed. The eyelids are
present in the shape of folds above and below the eye, and the different
parts of the auricula are distinguishable. By the end of the second month the
fetus measures from |
|
|
|
|
|
Third Month.—The head is
extended and the neck is lengthened. The eyelids meet and fuse, remaining
closed until the end of the sixth month. The limbs are well-developed and
nails appear on the digits. The external generative organs are so far
differentiated that it is possible to distinguish the sex. By the end of this
month the length of the fetus is about |
|
|
|
|
|
Fourth Month.—The loop of
gut which projected into the umbilical cord is withdrawn within the fetus.
The hairs begin to make their appearance. There is a general increase in size
so that by the end of the fourth month the fetus is from |
|
|
|
|
|
Fifth Month. |
|
|
—It is during this month that the first movements of the fetus are usually
observed. The eruption of hair on the head commences, and the vernix
caseosa begins to be deposited. By the end of this month the total length
of the fetus, including the legs, is from |
|
|
|
|
|
Sixth Month.—The body is
covered by fine hairs (lanugo) and the deposit of vernix caseosa is
considerable. The papillæ of the skin are developed and the free border of
the nail projects from the corium of the dermis. Measured from vertex to
heels, the total length of the fetus at the end of this month is from |
|
|
|
|
|
Seventh Month.—The pupillary
membrane atrophies and the eyelids are open. The testis descends with the
vaginal sac of the peritoneum. From vertex to heels the total length at the
end of the seventh month is from |
|
|
|
|
|
Eighth Month.—The skin
assumes a pink color and is now entirely coated with vernix caseosa, and the
lanugo begins to disappear. Subcutaneous fat has been developed to a
considerable extent, and the fetus presents a plump appearance. The total
length, i. e., from head to heels, at the end of the eighth month is
about |
|
|
|
|
|
Ninth Month.—The lanugo has
largely disappeared from the trunk. The umbilicus is almost in the middle of
the body and the testes are in the scrotum. At full time the fetus weighs
from six and one-half to eight pounds, and measures from head to heels about |
|
|
Note |
||
|
Bibliography |
||
|
|
||
|
BROMAN: Normale und abnorme Entwicklung des Menschen, |
|
|
BRYCE, TEACHER and KERR: Contributions to the Study of the Early Development and Imbedding of
the Human Ovum, |
|
|
HERTWIG, O.: Handbuch der Vergleichenden und Experimentellen
Entwicklungslehre der Wirbeltiere, |
|
|
HIS, W.: Anatomie menschlicher Embryonen, |
|
|
HOCHSTETTER, F.: Bilder der äusseren Köperform einiger menschlicher
Embryonen aus den beiden ersten Monaten der Entwicklung, |
|
|
KEIBEL and ELZE: Normentafel zur
Entwicklungsgeschichte des Menschen, |
|
|
KEIBEL and MALL: Manual of Human Embryology, |
|
|
KOLLMANN, J.: Handatlas der Entwicklungsgeschichte des Menschen,
|
|
|
KOLLMANN, J.: Lehrbuch der Entwicklungsgeschichte des Menschen, |
|
|
MALL: Contribution to the Study of the Pathology of the
Human Embryo, Jour. of Morph., |
|
|
MALL: Development of the Human Cœlom, Jour. of Morph., |
|
|
PETERS, H.: Ueber die Einbettung des menschlichen Eies und das
früheste bisher bekannte menschliche Placentationsstadium, |
|